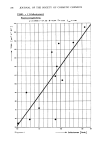
174 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 1977. This difference would be sufficient to change its Product Category Designation if this were a marketed product. The standard being tested was a 4.0% p-aminobenzoic acid (PABA) formulation prepared by Westwood Pharmaceutical Company, Buffalo, New York. The same 4% PABA standard formulation and an 8% homosalate (HMS) standard formulation prepared by Plough, Inc., Memphis, Tennessee had been used in a similar industry- wide study conducted in this country in 1977 to aid the FDA's OTC Review Panel on Topical Analgesics (the Sunscreen Panel) in determining whether uniformity of results could be achieved among independent testing laboratories and to assist in the Panel's selection of a standard sunscreen for use in conjunction with the proposed SPF testing procedures. The results of this study were distributed to the participants and are available on request from Plough, Inc., Box 377, Memphis, TN 38151. A review of our procedures indicated that the short wavelength cut-off filter of the solar simulator had been changed from a WG-305 filter, 2 mm thick to a WG-320 filter, !mm thick (Schott Glass designations). The new spectrum contained less short wavelength UV-B radiation than the previous one (Figure 1). These filters approximate the spectral differences between a southern (tropical) solar spectrum and a mid-latitude spectrum, respectively. The southern WG-305 spectrum was at the short wavelength limits of the proposed specifications as they were understood at the time and was replaced with a WG-320 filter, the type recommended by Berger (2) for solar simulator construction. 1.0 .2 WG- 305 WG-320 I I I I ! I I 280 300 320 340 360 380 400 Wavelength nm ! I Figure 1. Superimposed relative intensity spectra of the xenon arc solar simulator when equipped with two different short wavelength cut-off filters.
EFFECTIVENESS OF SUNSCREENS 175 The implications of this observation to standardized testing using solar simulators and to uniform labeling of sunscreening products are such that we immediately undertook a comparative study to confirm that the difference resulted from the change in spectrum. EXPERIMENTAL TEST MATERIALS 4% PABA standard sunscreen formulation prepared by Westwood Pharmaceutical Co., Buffalo, N.Y. 8% HMS standard sunscreen formulation prepared by Plough, Inc., Memphis, Tenn. (See reference 1.) SUBJECTS Only fair-skinned volunteers with skin types I, II, and III, having suitable areas of clear skin on their backs, were selected according to the Proposed Rules for Sunscreen Testing (1). Informed consent was obtained from each volunteer prior to testing. METHODS This was a paired study. Twelve volunteers were used in testing each of the standard sunscreen formulations with one of the formulations being applied to two comparable sites on the back of each volunteer. Determinations of SPF values were made with both the 2-mm WG-305 and the 1-mm WG-320 filter configurations of the same solar simulator on each volunteer, using the methods set forth in the FDA proposed rules (1). On the day prior to the test, each of the filters was used to administer a series of five UV light exposures of increasing duration to 1-cm 2 areas of unprotected skin closely adjacent to the appropriate test site to determine each volunteer's unprotected MED for that filter configuration of the solar simulator. These exposures were incremented according to the formula t x 1.25 n where n = the number of the exposure in the series and t = the duration of the first exposure. On the day of the test, one of the standard sunscreen formulations was applied uniformly at 2 /21/cm 2 to two areas of 50 cm 2, one on either side of the volunteer's back. A similar series of five exposures using each of the filters was given to the appropriate protected test site after a 15-min drying period. The duration of the exposures to the protected sites was determined from the unprotected MED for the appropriate filter and the anticipated protectiveness (SPF) of the test sunscreen formulation applied, incrementing in the same manner as above. The SPF value assigned to each formulation and filter configuration was based on the mean of the individual SPF values obtained in this test.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)