EFFECTS OF SURFACTANTS ON HAIR FRICTION 193 FIBER PRETREATMENT A second procedure provided additional evidence for less friction with more anionic on the hair surface. Fibers similarly bleached were soaked 5 minutes in 10% aluminum salt solution, rinsed and measured in 0.1% TEALS, providing a frictional coefficient of 0.18. Without aluminum pretreatment, friction was 0.41. Polyethyleneimine (PEI) also proved interesting for pretreatment effects. Fibers were soaked 5 minutes in 0.1% PEI at pH 10.2, rinsed and measured in 0.1% TEALS. PEI pretreatment reduced friction from 0.40 to 0.24. STRUCTURE OF ANIONICS By analogy to cationic sorption (31), greater retentivity and hence lower friction may be attainable by varying the chain length and hydrophilic groupings of anionic surfactants. Feasibility was tested by comparing decyl and hexadecyl alcohol sulfates (TEADS, TEACS) with a Dodecylbenzenesulfonate (TEADBS). In addition to neutral pH, TEADBS was also tested at pH 3.5 using acetate buffer. Each value shown in Table XIV is based on ten fibers, all from a single bleach treatment of one individual's hair. Table XIV Effect of Surfactant Structure Frictional Coefficients % AI TEADS TEACS TEADBS TEADBS (pH 3.5) 0.1 .44 .42 .45 .44 1.0 .33 .33 .38 .40 5.0 .27 .29 .32 .36 10.0 .25 .27 .31 .31 As for TEALS, increasing concentrations leads to lower friction. The C-10 and C-16 sulfates appear similar as tested, producing lower friction at high concentrations than neutral TEADBS. Evidently, much greater change in anionic structure would be required to achieve sufficient retentivity for meaningful decreases in friction. pH OF ANIONIC SOLUTIONS The TEADBS results at lower pH in Table XIV challenge our attempt to explain frictional effects. Anionic sorption was expected to increase with lower pH but friction failed to decrease. pH was investigated further by measuring friction in 0.1% sodium dodecane sulfonate (SDDS) buffered with acetate and lowered stepwise to pH 3 by HCI addition. Results in Table XV led us to question whether hard rubber mandrels were peculiar in some respect. Wool cloth was substituted and pH was raised stepwise by NaOH addition. Results with both substrates show friction passing through a maximum as pH changes. The problem needs further exploration but coincidence of the maximum with the
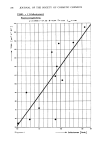
194 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table XV pH Effect with SDDS pH Hard Rubber (F.C.) pH Wool (F.C.) 6.9 .32 7.4 0.37 6.0 .37 5.1 0.44 5.0 .41 3.0 0.38 4.O .42 3.0 .38 isoelectric region of hair is suggested. Evidently, the assumption that more anionic sorbed produces lower friction must be qualified to include "at a given pH." ORGANIC COUNTERIONS Another approach to improving anionic surfactant substantivity is through the counterion. Sorption of CTAB increased with change in buffer counterion from acetate to citrate (31) and it seemed reasonable that anionic sorption could likewise be promoted by cations other than TEA or sodium. Properties such as aqueous solubility, activity coefficient, surface activity and CMC are known to be influenced by specific counterions, including tetra-alkylammonium ions (37,38). For convenience in screening trials, a salt is added to TEALS with sufficient interchange expected to permit detection of any specific effects on friction. In preliminary tests, tresses were shampooed with TEALS containing added salt or organic base and fibers from the tresses were measured in water. Tetra-ethylammo- nium bromide had no effect while diglycolamine and aminomethylpropanediol reduced friction slightly. Glucosamine (GCA) and dimethyloctylamine (DMOA) were added to TEALS with results summarized in Table XVI. Friction lowering is slight except for 0.13% DMOA Table XVI Effect of Organic Cations in TEALS • Amine or Cation % Added F.C. % Added F.C. None -- .42 -- -- GCA 0.1 .38 0.2 .37 DMOA 0.07 .38 0.13 .31 •0.1% TEALS at pH 7.2 which caused haze in the solution. Other counterions such as isopropanolamine, octyltrimethylammonium and aminomethylpropanol appeared ineffective. METALLIC CATIONS Information from shampoo experiments suggests that friction might be reduced for TEALS by addition of calcium ion or water hardness. The change in friction as increments of CaC12 are added to 1% TEALS is depicted in Figure 4. The initial rise to a maximum requires further testing since hard water may contain these quantities of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)