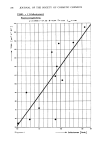
EFFECTS OF SURFACTANTS ON HAIR FRICTION 195 .4O .2O TEALS EQUIV. POINT I I I I I •1 I I I I ,, 2 4 6 8 I0 12 14 16 18 20 METALLIC ION ( M- MOLES / LITER} Figure 4. Effect of divalent metallic ions added to 1% triethanol ammonium lauryl sulfate (TEALS) at pH 7.5. calcium ion. The subsequent fall in friction is attributed to greater sorption as TEALS converts to the calcium salt. At the friction minimum, the mole ratio of Ca:TEALS is approximately 1:2 and further addition had no effect. Addition of magnesium ions to 1% TEALS, depicted in Figure 4, caused friction to decrease from 0.34 to 0.26. As for calcium, lowest friction coincides with a 1:2 mole ratio and more magnesium had no significant effect. The level reached with magnesium is somewhat lower and the solutions remain clear in contrast to turbid calcium solutions. Magnesium lauryl sulfate (MgLS) provided a slight edge, 0.32 vs. 0.34, over TEALS for friction and for wet combing of rinsed tresses. Friction is, however, higher than the 0.26 for magnesium added to TEALS. Since magnesium is known (39) to complex with triethanolamine, an equivalent of TEA was added to MgLS and pH was adjusted to 7.5. A friction of 0.26 was obtained. Tress combing failed to detect easier combing for MgLS when TEA was added at a 1:1 molar ratio. However, with 50% molar excess of TEA, combing scores did favor the complex system. Cuptic ion which forms strong complexes with TEA was investigated with results in Table XVII. One equivalent as cupric sulfate was added to TEALS and to SLS with pH adjusted to 7.5. The SLS became light blue and very cloudy while TEALS became dark blue and remained clear. Cuptic ion caused TEALS friction to increase and SLS friction to decrease. As a check, TEA added to the copper sulfate:SLS system caused friction to increase. The contrasting behavior of copper and magnesium with TEALS may be rationalized to an extent by considering published literature (39-41). Copper tends to coordinate
196 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table XVII Metal and Metal-TEA Complex Cations Additive TEALS SLS Cation Equiv. H. Rubber Wool H. Rubber -- 0 .34 .3O .38 Mg 1.0 .26 .25 -- Cu 1.0 .42 .41 .34 Cu 2.0 .40 -- .29 Cu/TEA 2.0/1.0 -- -- .38 A1 0.1 .27 -- -- A1 1.0 .19 .24 .31 AI/TEA 1.0/1.0 -- -- .21 through nitrogen, magnesium through oxygen. Assuming copper coordinates with amino groups and magnesium with hydroxyl groups of TEA, the Mg:TEALS complex has free amino groups for binding to keratin sites. Sorption experience indicates greater affinity for amino groups and accordingly Mg:TEALS may be more substan- tive to hair than Cu:TEALS. This explanation requires sorption data for support since other factors may also contribute to frictional differences between the two surfactant complexes. Aluminum ion effects on TEALS friction are summarized in Table XVII showing low friction. To gauge the importance of TEA, aluminum was added to SLS as potassium aluminum sulfate, pH was adjusted and friction determined as 0.31. Addition of TEA and again adjusting pH reduced friction to about the same level as for AI:TEALS. Cloudiness of the solution led to trial of aluminum at a 1/10 equivalent level. The solution was somewhat less cloudy and friction higher. Table XVIII Influence of Metallic Ions on TEALS Metal Classification Additive Appearance F.C. 1 2 CuSO4 Clear .42 B A-B CdSO 4 Clear .37 B B Hg(Ac)• Clear .36 B B Pb(Ac)2 Cldy, ppt. .34 -- A-B None Clear .33 (avg.) -- -- Zn(Ac)2 Cldy .31 -- A-B Zr(Ac)4 Cldy, ppt. .31 A A SnCl4 Cldy .29 A A CaCl= Cldy .29 A A SrCI= Cldy, ppt. .28 A A MgCI• Clear .24 A A K A1 (SO4)• Cldy, ppt. .19 -- A •Bailar, Reference 39 A--Bond to oxygen stronger. B--Bond to nitrogen stronger. •Pearson, Reference 40 A--Hard Lewis Acid. B--Soft Lewis Acid. A-B--Intermediate.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)