220 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS and Bauer (Stamford, Conn.) Hi-Flosil 60/200 mesh from Applied Science Lab Inc. anhydrous methanol, acetone and ethylene dichloride-all three distilled in glass from Burdick and Jackson sulfanilic acid, anhydrous sodium sulfate power, glacial acetic acid (aidehyde-free), hydrobromic acid, 48% and bromine, all A.R. grade from Baker or Mallinckrodt. All chemicals were used as received. Solutions used for analysis were prepared as follows: standard NDEIA solution--0.1% NDEIA in anhydrous methanol solution S is 0.75 gm sulfanilic acid plus 50 ml glacial acetic acid, q.s. to 250 ml with distilled water solution N is 0.3 gm 1-naphthylamine- 7-sulfonic acid plus 50 ml glacial acetic acid, q.s. to 250 ml with distilled water color solution is an equal volume of solutions S & N hydrobromic acid bromine solution use 62.5 ml of 48% hydrobromic acid plus 2 ml bromine, q.s. with glacial acetic acid to i liter. Store all solutions in dark or brown bottles and cap tightly since the nitrogen oxides in the air develop the color. Do not use color solution unless it is colorless. The glassware is all initially treated with 6 N hydrochloric acid to remove adsorbed nitrogen oxide from its surface and then put thru the entire assay process before actually being used for assays. Bottles and vials remained sealed until the day they are to be used. Before use, bottles and vials are cleaned by rinsing at least three times each with tap water, then distilled water and finally with acetone and then dried in the vacuum oven. The column is prepared by using a wad of glass wool, 10 gm Hi Flosil is added and gently tamped to level the surface, 25 gm anhydrous sodium sulfate is added. The assembled column is washed with 200 ml of acetone before use. METHOD OF ANALYSIS The assay procedure utilizes the well known Griess Test as modified by Ilosvay for nitrite using Cleve's acid (1-naphthylamine-7-sulfonic acid) (1) which is similar to the color procedure used in the nitrite method authored by Rosenberg, et al. (2). By increasing sample size one is able to achieve a sensitivity of better than one part per billion. Thus with a sample size of 2.5 gm a sensitivity of 3-5 ppb is attained. Further sensitivity is proportional to increased sample size. A 5-gm sample plus 2.5 ml of water is extracted and concentrated--using 100 ml of acetone for extraction. Extraction is performed by placing the sample flask into an ultrasonic bath for 30 minutes. Sulfanilic acid (0.25 gin) is added to the acetone-water during extraction to destroy any nitrite present. This extract is dried and cleaned up by passing it thru an acetone prewashed anhydrous sodium sulfate-silica gel column. The 125 ml of acetone extract which is collected is divided in half. One sample of each pair is "spiked" with 5 ug NDEIA to: 1) determine recovery, 2) be certain nothing is present in the extract that prevents color development. One milliliter of glacial acetic acid is added to each portion of the acetone extract to insure destruction of any nitrite that may not have been earlier destroyed (3,4,5). Each portion is evaporated to dryness by means of a steam bath in a 4 oz. screw cap bottle. The extract is taken to dryness in a vacuum oven heated at 90øC with at least 125-250 torr (20-25 inches of vacuum). Since additional cleanup is needed, the residue is dissolved in one milliliter of water and this solution is extracted with 10 ml of ethylene dichloride. The ethylene dichloride is removed by means of a Pasteur pipette. The aqueous extract is taken to dryness in the vacuum oven. To the residue in the 4 oz.
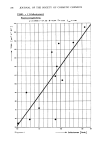
NITROSAMINE DETERMINATION 221 Table I Comparison of Thermo Electron Corp.'s with Max Factor's Results. Thermo Electron Corp. Sample Results NDE1A Found Max Factor's Results Total Nitrosamine as NDE1A a 3200 ppb 2600 ppb b 1400 1900 c 180 270 d 60 40 bottle, 10 ml of ethylene dichloride are added. This is a "keeper" solvent. This is necessary to prevent a volume change of the color solution. Then we place an 8-cc vial containing 2 ml of color solution into the 4 oz. bottle. To the 4 oz. bottle 7 ml of 3% hydrobromic acid with 0.2% bromine in glacial acetic acid is added taking care none of this gets into the 8-cc vial. The bottle is quickly capped using a cap with a teflon or polyethylene liner. The volatile nitrosyl bromide or nitric oxide formed (3,5) diffuses over into the color vial. Thus we have concentrated our entire amount of isolated nonvolatile "nitrosamine" from the 2.5 gm sample into 2 ml of color solution. Walters has shown this reaction to be quite specific for nitrosamines (4). A recent paper by Fine shows that we may have either N-nitroso or C-nitroso compounds (5). However, he pointed out that C-nitroso compounds are so unstable in most cases that they have yet to be reported as present in the environment. We allow the unit to stand at room temperature in the dark for two days (optimum time found). The color vial is removed and its absorbance at 520-5 nm is measured. Blank and standards must be run daily. We have found the method successful on cosmetic products, raw materials, grilled hot dogs, cutting fluids, and beer. We have assayed samples sent to Thermo Electron Corporation and have found good correlation with their findings. Our technique suffers the same problem as Thermo Electron Corp's procedure in that loss of the nitrosamine does occur during the isolation procedure and we are working to improve this. The same procedure is used for "nitrite" determination: One gram of most raw materials is added directly to the 4 oz. bottle and you proceed from the point of adding the ethlyene dichloride as the "keeper" solvent. Eighteen to twenty samples plus standards can be run by one person in eight hours. Table II Example of Calibration Curve Using NDE1A Concentration of NDE1A (ug) Absorbance 0 .024 1 .185 2 .248 3 .362 4 .445 5 .613
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)