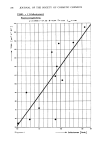
222 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS DISCUSSION We have modified Walter's denitrosating solution, (i.e. 3% Hydrobromic acid in glacial acetic acid), when learned that: 1) aged solutions were yielding higher amounts of color 2) that bromine denitrosates nitrosamine (6). With this modification of adding 0.2% bromine to the hydrobromic acid in glacial acetic acid we increased our color or sensitivity 3-to 4-fold. We have not tried to identify what nitrosamine is actually present, but further work can be done with the sample after the color vial is removed since the sample has not been contaminated. The resulting amine(s) that are present probably can be identified by following Eisenbrand's procedure of forming either the heptafluorobutyryl or dansyl chloride derivative of the remaining amine(s) (7). REFERENCES (1) L. C. Thomas and G.J. Chamberlin, Nitrite Method I in Colorimetric Chemical Analytical Methods 8th ed., The Tintometer Ltd., Salisbury, England. p281 (1974). (2) I.E. Rosenberg, J. Gross, T. Spears, and U. Caterbone, Methodology development for the determination of nitrite and nitrosamines in cosmetic raw materials and finished products. J. Soc. Cosmet. Chem. 30, 127-135 (May/June 1979). (3) M. J. Downes, M. W. Edwards, T. S. Elsey, and C. L. Walters, Determination of a non-volatile nitrosamine by using denitrosation and a chemiluminescence analyser, Analyst 101, 742-748 (Sept. 1976). (4) E. M. Johnson and C. L. Walters, The specificity of the release of nitrite from N-nitrosamines by hydrobromic acid, Analytical Letter, 4 (6), 383-386 (1971). (5) I. S. Krull, E. U. Goff, G. G. Hoffman, and D. H. Fine, Confirmatory methods for the thermal energy determination of N-nitroso compounds at trace levels, Anal. Chem. 51, 1706-1709 (Sept. 1979). (6) R. F. Eizember, K. R. Vogler, R. W. Souter, W. N. Cannon, and P.M. Wege II, Destruction of Nitrosamines. Treatment of nitrosamines with various acids and halogens, j. Org. Chem. 44 (5), 784-786 (1979). (7) G. Eisenbrand, Determination of volatile nitrosamines at low levels in food by acid-catalysed denitrosation and formation of derivatives from the resulting amines. IARC Scientific Publication No. 3, P. Bogovski, R. Preussmann, E. A. Walker and W. Davis, Eds., International Agency for Research on Cancer, Lyon (1972) pp 64-70.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)