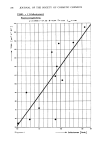
EFFECTS OF SURFACTANTS ON HAIR FRICTION 191 evidently change from electronegative to neutral to electropositive, relative to the hard rubber surfaces, depending on the amount of cationic present. Friction reduction is in large part due to non-polar long chains but, as for sorptivity and static, the polar ends of sorbed molecules may also play a direct role. RINSE TIME Fibers were measured in 0.1% SAAC solution at pH 3.6 and then remeasured in water as a function of time. Results depicted in Figure 3 indicate that excess surfactant is removed with water to minimize friction and further removal is extremely slow. A 0.5 0.4 0.2 0.1 • MEASURED IN O. I ø/o SOLUTION "'Oo0 • o o o 0 I I I I I ' 55 I I I 50 I00 150 200 •0 1200 MINUTES IN I•ATER Figure 3. Single fiber measurements in 0.1% stearalkonium chloride (SAAC) at pH 3.6, followed by measurements in water. portion of surfactant must remain on the hair surface despite prolonged soaking with perhaps some replenishment by sub-surface surfactant. The experiment was repeated with similar results for DMSA at pH 3.6. IMMERSION TIME IN SOLUTION Fibers immersed in cationic solutions were measured after a few minutes and at 20 hours, for the data in Table XII. Each solution was buffered at pH 3.6 with acetate. Frictional changes for most cationics appear small indicating that immersion time is a minor factor in friction determinations. This was anticipated since friction is primarily a surface property. Sorption of surfactants reaches equilibrium in a short time at fiber
192 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table XII Immersion Time in Solution Fog. Cationic % Concentration 2-10 Minutes 20 Hours CTAB 0.100 .39 .40 CTAB 0.010 .30 .25 SAAC 0.100 .47 .49 DMSA 0.100 .39 .46 FPAC 0.100 .19 .20 FPAC 0.010 .17 .21 surfaces and then increases at a diminishing rate as interior sites become occupied (31). III. ANIONIC SURFACTANTS An important difference between currently used anionic and cationic surfactants is in their relative affinity to hair. Typically, cationics sorb strongly, resist desorption and produce low frictional coefficients. This suggests that if sufficient anionic surfactant were retained on hair, easier combing and lower friction might result. ANIONIC CONCENTRATION A usual means for increasing sorption is to increase the surfactant concentration. The preceding work, in conjunction with cationic studies, indicates that, for anionics, fiber friction does decrease as solution concentration is raised. To confirm this, 10 fibers were measured at increasing concentrations of TEALS and then in water. In a second experiment, separate groups of 10 fibers were measured at each TEALS concentration. Fibers were from one individual (LAL) but were separately bleached for each experiment. Results in Table XlII show a decrease in friction with increasing concentration. When Table XIII Concentration Effects % TEALS Water 0.1 1.0 5.0 10.0 20 Hrs. 48 Hrs. Exp. 1 -- .39 .32 .29 .33 .34 Exp. 2 .41 .32 .24 .25 -- -- the first set was remeasured in water, friction increased as expected from desorption considerations. Combing tests on bleached hair tresses treated with TEALS confirm that unrinsed tresses are easier to comb than rinsed tresses. In 0.1% FPAC solution, friction of fibers from the second experiment fell to 0.14, indicating that even at 10%, TEALS is less effective than a good cationic in agreement with combing.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)