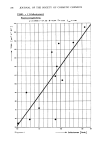
172 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS O0 0:1 0".2 0:3 CO NCE NT R A TION (%) Figure 6. Preservative death time curve for S. aureus in lotion containing different concentrations of glyceryl monolaurate. ACKNOWLEDGEMENTS I thank Bill Dickman and Joan Barger for their assistance in preparing the test samples. REFERENCES (1) Anon, "Microbiological tests, antimicrobial preservatives--effectiveness," United States Pharmacopeia XIX, The United States Pharmacopeial Convention: Rockford, Md., 1975 pp 487-592. (2) Preservation Subcommittee of the CTFA Microbiological Committee, A guideline for the determina- tion of adequacy of preservation of cosmetics and toiletry formulations, TG/I Cosmet. Journal, 2, 20-23 (1970). (3) D. S. Orth, Linear regression method for rapid determination of cosmetic preservative efficacy,J. Soc. Cosmet. Chem., 30, 321-331 (1979).
J. Soc. Cosmet. Chem., 31,173-177 (July/August 1980) Sunscreen product effectiveness can vary with different simulated solar ultraviolet spectra GORDON J. LEVEE, ROBERT M. SAYRE, and EDWARD MARLOWE, Plough, Inc., P.O. Box 377, Memphis, TN 38151. Received December 5, 1979. Synopsis Two different short wavelength cut-off filters were used in a xenon arc SOLAR SIMULATOR employed in SUNSCREEN PRODUCT EFFICACY testing. Different efficacies were indicated for a standard formulation, depending on the filter used. These data document the importance of the short wavelength characteristics of the ULTRAVIOLET SPECTRUM used in tests for product labeling. A narrower definition of solar simulator specifications for sunscreen product testing may be necessary. INTRODUCTION In August of 1978 the United States Food and Drug Administration (FDA) published proposed rules to establish conditions for the safety, effectiveness and labeling of over-the-counter (OTC) sunscreen drug products (1). Included among the proposed rules and label requirements are methods for determining a Sun Protection Factor (SPF) which is a measure of sunscreen product effectiveness. An SPF value is defined as the amount of UV energy required to produce a minimal erythema dose (MED, that amount of energy which produces a minimally detectable redness) on sunscreen- protected skin divided by the amount of UV energy required to produce an MED on unprotected skin. In the case of solar simulators, where the energy emitted is assumed to be constant, exposure time is commonly used to determine the MED. A solar simulator is recommended as the preferred source of ultraviolet (UV) energy for these SPF determinations because natural sunlight is extremely variable and uncontrollable. The proposed rules specify that a solar simulator shall have a "continuous emission spectrum in the UV-B (290-320 nanometers) with less than one percent of its total energy contributed by ... wavelengths shorter than 290 nanometers" and "not more than five percent of its erythemically effective energy be contributed by nonsolar wavelengths" (1). Many commercial sunscreen products already bear SPF designations in anticipation of the finalization of these FDA rules. Recent observations made in our laboratory indicate that a narrower definition of the solar simulator specifications may be desirable. While testing a standard sunscreen formulation in connection with an international comparison of sunscreen testing methods, we found that the SPF values we obtained were only 70% as great as those obtained when this same standard had been tested in 173
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)