EFFECTS OF SURFACTANTS ON HAIR FRICTION 187 II. CATIONIC SURFACTANTS Distinction of cationic from anionic surfactants was relatively simple throughout most earlier experiments with lower wet friction for cationics under a variety of test conditions. This distinction narrows and at times disappears as conditions and compositions are varied. However for practical purposes, it is valid to say that cationic surfactants produce low friction and anionic surfactants high friction. MEASUREMENTS IN WATER Distinction among cationic surfactants and their compositions was more difficult, requiring bleached fibers and hard rubber mandrels to enlarge cationic differences. Effects of bleaching hair fibers and rubbing on various substrates are illustrated in Table VII using cetrimonium bromide (CTAB) and SLS as reference surfactants. Table VII Measurements in Water Fibers Pre-Treat Steel Wool H. Rubber DM CTAB .29 .26 .31 DM, BL CTAB .35 .28 .42 DM SLS .25 .30 .32 DM, BL SLS .38 .32 .53 High friction for CTAB-treated fibers, approaching that for SLS, was ascribed to immersion in water which allowed greater desorption of CTAB (31) than would occur during normal rinsing and wet combing of hair. MEASUREMENTS IN 0.1% SOLUTION To avoid excessive desorption, friction was measured in solutions of surfactants at a low active ingredient (AI) concentration. In Table VIII, cationics, known to be effective for wet combing, are compared to CTAB and SLS using 0.1% AI solutions and then water as measuring media for bleached fibers on hard rubber. Results/•n Table VIII obviously do not fit practical experience. Only the fatty amido/-propylammonium chloride (FPAC) is low in friction and remains low when measured in water. The friction increase with CTAB and SLS fibers in water can be explained by desorption but behavior of stearalkonium chloride (SAAC) and dimethyl- Table VIII Measurement in 0.1% Solutions Surfactant, F.C. SLS CTAB DMSA SAAC FPAC 0.1% AI Soln. .39 .39 .39 .47 .19 Water .54 .46 .31 .30 .17 •Buffered to pH 3.6 with acetate. 2Same fibers subsequently measured in water.
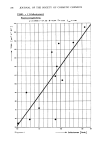
188 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS stearamine (DMSA) was puzzling. At 0.1% AI, values are comparable to SLS and, with the extreme rinsing imposed by water measurements, friction decreases although not to levels expected on the basis of practical combing. CONCENTRATION EFFECTS The friction test appears suited for acquiring fundamental information leading to a better understanding of frictional effects but a practically oriented set of test conditions must also be provided to predict results of use tests. Obviously preceding experiments have not satisfied this objective but have emphasized concentration of surfactant at the rubbing interfaces to be an important test variable. Evidently for certain cationics, friction passes through a minimum as concentration is reduced on the hair surface. To better define concentration effects and especially friction minima, bleached fibers were measured in buffered solutions at various low surfactant concentrations. Results are complied in Table IX and graphed for CTAB in Figure 2. Table IX Concentration Effects % Concentration, F.C. Cationic pH-Buffer 0.20 0.10 0.05 0.01 0.005 0.001 01 CTAB 3.6 Acetate -- .39 .38 .30 .25 -- .54 CTAB 3.6 Citrate -- .36 -- .28 .25 -- -- CTAB 9.3 Carbonate .34 .30 .31 .28 .35 -- .49 DMSA 3.6 Acetate -- .39 .41 .32 .31 .29 .54 SA 3.6 Acetate -- .39 -- -- -- .21 .54 SAAC 3.6 Acetate -- .45 .40 .39 -- .28 .54 Q-18 3.6 Acetate -- .17 -- -- -- .19 .54 FPAC 3.6 Acetate .19 .19 -- .17 -- .27 .54 1Fibers in .02 M buffer solutions, no surfactant. Friction minima are evident for CTAB under the test conditions. Exchange of citrate for acetate buffer had no significant effect although sorption is known (31) to increase marginally with citrate buffer. Replacing hard rubber with wool produced a lower curve for CTAB but with a similar minimum. Both DMSA and SAAC produce minima and stearamine (SA) which appeared in combing tests to have an advantage over the tertiary amine DMSA, also shows a minimum but at a lower friction value. The two higher molecular weight surfactants, Quaternium-18 (Q-18) and FPAC in Table IX have low friction at all test concentra- tions. Additional data is needed to detect reliably whether friction minima exist for these two surfactants. Results of sorption (31) are pertinent to the frictional behavior of these cationics. CTAB for example sorbs in relatively large amounts to hair with appreciable penetration into the fibers but, upon dilution of the contact solution, readily desorbs. Unreported data (33) show that cationics such as SAAC sorb at lesser amounts, penetrate more slowly, but have greater resistance to desorption. Other cationics, for
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)