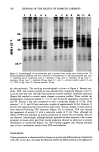
AMINO ACID METABOLITES IN DRY SKIN 195 ridges were indistinct, and scales were visible. These facts suggest that the etiology of the superficial lesion of dry skin may partly be interpreted as the stimulatory effects of low temperature and dry air in winter. These cause a slight inflammation of the skin, accelerating keratinization, leading to abnormal stratum corneum. Dry skin encountered in winter may involve only the surface of the stratum corneum without changes in viable epidermal levels. Although it is also certain in the present study that parakeratosis could not be demonstrated in some cases in which roughness of the skin was macroscopically evident, examination with an increasing number of panels revealed changes in free amino acid compositions similar to those noted in hyperkeratotic epidermis. In addition, parakeratosis was increasingly noted in dry skin. All of these results suggest that changes in the epidermis take place in dry skin. Based upon this assumption, in order to obtain a good treatment for chapped skin, it is necessary to consider improvement of the lesion in the viable epidermis. REFERENCES (1) P. Flesh, C. Hodgson, and E. C. J. Esoda, Water-soluble organic components of psoriatic scales, Arch. Dermatol., 85, 84-92 (1962). (2) E. Schwarz, Freie aminosauren und hornstoff in psoriatischen und normalen epidermalen verhor- nungsprodukten, Arch. Klin. Exp. Dermatol. 225, 229-305 (1966). (3) S. Marstein, E. Jellurn, and L. Eldjarn, Reduced amounts ofpyroglutamic acid in scales from psoriatic plaque, Arch. Dermatol., 108, 578-579 (1973). (4) J. D. Rossmoller and W. G. Hoekstra, Hexadecane-induced hyperkeratinization of guinea pig skin. IV. A comparison of free amino acid levels in normal and hyperkeratotic epidermis, J. Invest. Der- matol., 47, 44-48 (1966). (5) K. Nakamura, Y. Morikawa, and I. Matsumoto, High speed liquid chromatographic analysis of citric, lactic, urocanic and pyroglutamic acids in cosmetic products, Bumeki Kagaku, 29, 314-318 (1980). (6) I. Horii, S. Akiu, K. Okazaki, K. Nakajima, and S. Ohta, Biochemical and histological studies on the stratum corneum of hyperkeratotic epidermis, J. Soc. Cosmet. Chem. Japan, 14, 174-178 (1980). (7) I. Horii, K. Kawasaki, J. Koyama, Y. Nakayama, K. Nakajima, K. Okazaki, and M. Seiji, His- tidine-rich protein as a possible origin of free amino acids of stratum corneum, J. Dermatol., 10, 25-33 (1983). (8) N. W. DeLapp and D. K. Dieckman, Biosynthesis of pyrrolidone carboxyric acid in hairless mouse epidermis, J. Invest. Dermatol., 68, 293-298 (1977). (9) Unpublished observations. (10) H. Goldschmid and A.M. Kligman, Exfoliative cytology of human horny layer, Arch. Derm., 96, 572-576 (1967). (11) H. Goldschmid and M. A. Thew, Exfoliative cytology of psoriasis and other common dermatoses, Arch. Derm., 106, 476-483 (1972).
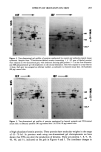
J. Soc. Cosmet. Chem., 35, 197-205 (July 1984) Effects of the chemical irritants anthralin and benzoyl peroxide on mouse skin epithelial cell protein production CHRISTOPHER J. MOLLOY, MICHAEL A. GALLO, and JEFFREY D. LASKIN, Department of Environmental and Community Medicine, UMDNJ-Rutgers Medical School, Joint Program in Toxicology, Rutgers University, Piscataway, NJ 08854. Received November 15, 1983. Presented at the Society of Cosmetic Chemists Annual Meeting, New York, December 1, 1983. Synopsis The widely used topical agents anthralin and benzoyl peroxide are potent skin irritants. In the mouse skin model these compounds produce hyperplasia and are effective skin tumor promoters. In the present studies, we have examined the effects of anthralin and benzoyl peroxide on epidermal proteins and compared them with changes produced by the potent phorbol ester tumor promoter 12-O-tetradecanoyl-phorbol-13-acetate (TPA). All compounds were applied topically to the shaved dorsal skin of mice. Twenty-four hours later skin fragments were pulse-labeled with 35S-methionine to assay total protein production. Anthralin and TPA produced a marked inhibition of protein synthesis when compared to control and benzoyl peroxide- treated skin. When skin was treated with anthralin at a promoting dose (80 }xg, 354 nmole), incorporation of the radiolabel into epidermis was only 22% of control. Promoting doses of TPA (10 }xg, 17 nmole), however, inhibited protein synthesis to 63% of control. Qualitative changes in protein production induced by the chemicals were examined using one- and two-dimensional gel electrophoresis. Using these tech- niques, we were able to detect over 100 individual proteins from both treated and untreated mouse skin. TPA and anthralin were found to alter the production of at least seven distinct proteins, six of which are keratins. The profile of benzoyl peroxide-treated epidermal proteins, however, resembled those of control skin, suggesting that this chemical promoter may act by a mechanism distinct from TPA and anthralin. INTRODUCTION Anthralin (1,8-dihydroxy-9-anthrone) and benzoyl peroxide are common pharmacologic agents employed in the topical treatment of various skin disorders. Anthralin is active against psoriasis and has been used clinically over the past 50 years (1). Benzoyl peroxide is a common ingredient in proprietary acne formulations (2). Both compounds, while structurally quite dissimilar (Figure 1), have in common the ability to cause skin irritation and tumor promotion in the two-stage mouse skin carcinogenesis assay (3,4). Tumor promoters do not cause cancer alone, but act to facilitate the expression of the altered tumor cell phenotype through mechanisms as yet unclear (for a review, see Weinstein, et al., ref 5). The most potent chemical of this class is the phorbol ester, 12-O-tetradecanoyl-phorbol-13-acetate (TPA), derived from croton oil (6). Application 197
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)