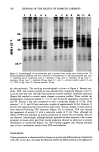
ANIONIC SURFACTANT QUANTITATION BY FTIR 219 Table V Unknown Raw Material Samples vs. Corresponding "Assayed-Sample Standard" Raw Materials FTIR Using Using Solvent- Titration Unsubtracted Subtracted Sample Abs Value Abs Value Analyst # 1 Analyst #2 Sodium lauryl sulfate Ammonium lauryl sulfate Alpha olefin sulfonate Sodium laury! ether su!fate Sodium lauryl diether su!fate 28.08 27.96 28.00 29.3 28.08 27.94 27.73 27.76 28.26 28.0 27.80 27.86 38.37 38.54 39.08 41.0 38.19 38.30 24.14 23.89 24.91 24.65 24.01 23.70 24.68 24.63 26.13 -- 24.83 24.83 Table VI Unknown Finished Product Samples vs. "Assayed-Sample Standard" Finished Product (Finished Product Containing Sodium Lauryl Ether Suloeate) Sample # FTIR Using Using Titration Unsubtracted Solvent-Subtracted Abs Value Abs Value Lab. # 1 15.04 ) • = 15.04 15.17 ) -- 14.49 15.03 15.14 X = 15.16 14.94 15.04 (y = 0.006 15.17 (y = 0.017 -- 15.03 14.94 ) -- 14.99 ) X = 15.11 14.94 X = 15.01 14.97 14.90 15.15 o' = 0.121 15.37 o' = 0.225 -- -- 15.06 15.13 ) X = 15 20 15.32 ) X = 15.44 3 15.26 ' 15.55 15 35 15.21 (y = 0.066 15.46 (y = 0.116 ' -- 15.16 -- 15.03 15'04 ) X = 15.08 ) X = 15.23 4 15.11 15.29 14.78 15.08 (y = 0.035 15.24 (y = 0.066 15.23 An additional advantage of the FTIR method is the capability of storing the "standards" on a floppy disk. Any time a "standard" need be run for quantitation or for qualitative fingerprinting, it is easily accessed by recalling it from the floppy back into an instru- ment file. This recalling procedure takes about five seconds and eliminates the need to rerun the "standard" each time an analysis is required. Extensive work has been done investigating how instrumental variations such as changes in throughput might affect
220 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS spectral storage and recall as applied to quantitative analyses. To date, all results obtained have verified the acceptability of floppy disk storage. In conclusion, the method of surfactant quantitation presented here has proven to be a viable alternative to the mixed indicator titration procedure. The FTIR method is simple, accurate, precise, and easily applicable to both raw material and finished product surfactant samples. Presently, we are investigating the extended use of the CIRCLE and FTIR techniques for a wide variety of cosmetic raw materials and finished products. REFERENCES (1) S. R. Epton, A rapid method of analysis for certain surface-active agents, Nature [London], 160, 795-796 (1947). (2) S. R. Epton, A new method for the rapid titrimetric analysis of sodium alkyl sulphates and related compounds, Trans. Faraday Sot., 44, 226 (1948). (3) J. W. Reid, G. F. Longman, and E. Heinerth, Determination of anionic-active detergents by two- phase titration, Tenside, 4 (9), 292-304 (1967). (4) American Society for Testing and Materials, Standard test method for synthetic anionic ingredient by cationic titration, Designation D3049-75. (5) J. W. Jenkins and K. O. Kellenbach, Identification of anionic surface active agents by infrared absorption of the barium salts, Anal. Chem., 31 (6), 1056-1059 (1959). (6) R. Matissek, Combination of thin-layer chromatography and infrared spectrometry for the identifi- cation of ethoxylated and non-ethoxylated alkyl sulfate surfactants, Parfumerie und Kosmetik, 64, 59- 64 (1983). (7) S. Hashimoto, H. Tokuwaka, and T. Nagai, Determination of c•-olefin sodium sulfonate, linear alkylbenzene sodium sulfonate and sodium alkyl sulfate in detergents by infrared spectroscopy, Bunseki Kagaku, 22 (5), 559-563 (1973). (8) N. B. Colthup, L. H. Daly, and S. E. Wiberley, Introduction to Infrared and Raman Spectroscopy, (Academic Press, NY, 1964), p 409. (9) R. M. Silverstein, G. C. Bassler, and T. C. Morrill, Spectrometric Identification of Organic Compounds, 3rd ed., (John Wiley & Sons, Inc., New York, 1974), pp 73-119.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)