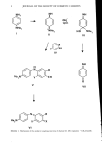
STRATUM CORNEUM HYDRATION 11 z uJ I- z o 0.5 0.4 0.3 0.2 0.1 I I I / I I I I 0 20 40 60 80 100 RELATIVE HUMIDITY (%) Figure 1. The mass of water absorbed per mass of dry stratum corneum as a function of ambient relative humidity. All experiments were performed with human samples at 25øC. Error bars represent +_ SEM of multiple determination at each relative humidity. obtained at 25øC showed that the SC behaves as though it were composed of material of 300 to 500 molecular weight. Alternatively, the reciprocal of the molecular weight determined in this manner represents the average number of water binding sites per gram of SC. Jacques' analysis of the data of Spencer et M. (8) by this method showed that the number of water binding sites doubled when the SC was heated from 20 to 35øC. Similar results were obtained by either Langmuir or BET isotherm analyses of these data, leading Jacques to conclude that the number of water binding sites increased with increasing temperature of the SC, possibly due to thermally induced protein un- folding. Thus, SC-water binding isotherm data can be interpreted by two models. The first suggests that the enthalpy, or strength of binding of a constant number of sites, decreases with increasing water content of the SC, while the second model suggests that the number of constant enthalpy binding sites increases with increasing temperature. It is quite likely that in reality, both the number and strength of water binding sites in the SC change in response to changes in temperature and water content.
12 JOURN_•,L OF THE SOCIETY OF COSMETIC CHEMISTS The kinetics of water uptake were also gravimetrically determined by Anderson et al, (7) who investigated sorption and desorption at constant temperature and RH as a function of time. Their results showed an initial, rapid water uptake with a rate constant de- pendent on the water content, followed by long-term absorption with a rate constant independent of the water content. When combined with their equilibrium binding data, these results suggested two distinct classes of bound water. At low content, water was rapidly and tightly bound. In contrast, further increases in water content resulted in slower uptake and weaker binding. An alternative method of measuring in vitro hydration of SC involves spectrometric techniques. In addition, the results of these methods provide unique insight into the molecular mechanisms of water binding to the SC. Hansen and Yellin (14) employed both infrared (IR) and nuclear magnetic resonance (NMR) techniques to investigate the mechanism of water uptake by human SC. From IR measurements, the relative contri- bution of three distinct classes of water were assessed as a function of water content. Results show that below about 10% (w/w) hydration, water was tightly bound to the polar sites of the SC proteins (primary hydration). Water was less tightly bound (prob- ably hydrogen bonded to the primary hydration water) at water contents from 10 to 40% (w/w). At a water content above about 50% (w/w), water absorbed by the SC had properties more like that of the bulk liquid. The NMR relaxation times of water were also measured for SC samples at varying water contents. These results suggest that molecular motion of water in the SC is characterized by at least two time constants. At low hydration the water had low mobility, while at concentrations above about 30% (w/w) water was of higher mobility, much like that of bulk water. In summary, in vitro hydration of human SC has been measured gravimetrically and spectrometrically for samples prepared by trypsin digestion of excised human skin. The results show that the extent and mechanism of water uptake by the SC varies dramati- cally with water content. At low water content, water is rapidly adsorbed and tightly bound, most likely to the polar groups of the protein keratin. In contrast, at higher water contents, water uptake is slower and added water is in a liquid-like state. WATER LOSS The mechanism of water flux through the SC is best investigated with in vitro samples. In general, these experiments involved the attachment of full thickness skin or trypsin- isolated SC, SC side out, to a container of water or aqueous buffer. Loss of water to the surrounding atmosphere (at controlled temperature and RH) was then determined ei- ther by mass change (6) or by water uptake of the ambient atmosphere (16,17). Alter- natively, the flux of radiolabelled water through SC maintained at constant water con- tent and temperature was measured (18). Results of early experiments showed that the primary barrier to water loss in the skin is located in the SC. For example, removal of the SC from intact human skin caused an approximate 100-fold increase in water flux, to a value approaching that of free water diffusion. In contrast, the removed piece of SC had a lower flux, equivalent to that of intact skin (14). Water loss through the SC is a passive diffusion process described by Fick's Law (see above) which predicts that flux depends on water concentration, SC thickness, and the diffusion and partition coefficients of water in the SC. Numerous investigators have
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)