36 jOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS cessful for the separation of human epidermis (6,7), embryonic, or newborn animal skin (8,9). However, no suitable method for separating young or mature animal skin has been described. Unlike epidermal membranes prepared for biochemical or biological evaluation, a fun- damental requirement for prepared epidermal sheets for in vitro permeability measure- ments is an intact stratum corneum with unchanged diffusional properties. To develop a technique for rat skin, we examined a variety of published methods: heat treatment (10) enzymic digestion with trypsin (11) exposure to dithiothreitol [DTT] (8) and soaking in aqueous solutions of calcium chloride and sodium bromide. This paper describes a modified technique using sodium bromide solution to prepare intact epidermal sheets and the assessment of the permeability properties of the sepa- rated epidermal membranes using a small polar molecule ([3H] water), a permanently charged divalent cation ([•4C] paraquat), and a lipophilic molecule (toluene). MATERIALS AND METHODS ANIMALS Albino (Wistar-derived) Alderley Park strain rats, of either sex, 1 day to 8 weeks old were used in this study. They were anaesthetized by exposure to "Fluothane" (Halo- thane BP ICI PLC, Pharmaceuticals Division, UK) and killed by cervical dislocation. The dorsal and flank skin was lightly trimmed, removed, and stored at 4øC until needed. HUMANSaN Abdominal skin (primarily female) was obtained from cadavers, normally 60 years of age or older. Subcutaneous fat and muscle were removed and the tissue stored on alu- minum foil at 4øC until needed, but for a maximum of 7 days. SEPARATION TECHNIQUES Human and rat whole skin (epidermis plus dermis) samples were immersed in the following solutions (Table I): 1 M and 2 M sodium bromide (NaBr BDH Chemicals Ltd, Poole, Dorset, UK) 0.05 %, 0.1% and 0.5 % w/v trypsin type II (in physiological saline) from porcine pancreas (Sigma Chemicals, London) 0.05% w/v hyaluronidase (in saline) type 1-5 enzyme from bovine testes (Sigma Chemicals) 0.1 M dithiothreitol (DTT, Cleland Reagent DL-DTT supplier Sigma Chemicals) 2 M analar calcium chlo- ride (CaCI2 supplier Hopkins and Williams, Essex, UK) for various time periods at a range of temperatures. After this, attempts were made to peel the epidermis from the dermis. CHEMICALS [3HI water (supplied by Radiochemical Centre, Amersham International PLC, UK) was diluted with physiological saline to a final specific activity of 5 I-tCi/ml [•4C] paraquat dichloride (Radiochemical Laboratory, Billingham, ICI PLC, Teesside, UK) was di-
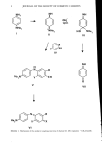
PRODUCTION OF INTACT EPIDERMAL MEMBRANES 37 Table I Details of the Chemical Solutions and Conditions Used to Allow Epidermal Membrane Production Rat Immersion Age Temp Time Solution Concentration (weeks) øC (hr) Observations Sodium bromide 1 M 2M Calcium chloride 2 M Dithiothreitol 0.1 M 1 0.1M Trypsin 0.05 % w/v 0.1% w/v 0.5% w/v Hyaluronidase 0.05 % w/v 7-8 20 18-24 No separation possible. 7-8 37 3-4 Epidermis at edges of skin removable. 17-18 Epidermis fragile when touched. 4- 5 20 24 Epidermal membranes could be separated from dermis. 7-8 20 24 } No apparent separation 4- 5 20 24 of epidermal membrane possible. day old 4 2 Separation of epidermis from dermis possible. 7-8 4 2 No separation of epidermis. 7-8 4 24 No separation possible. 7-8 37 10-12} Apparent tissue digestion. 7-8 37 10-12 7-8 4 24 No separation possible. luted with physiological saline to a final activity of 8 IxCi/ml toluene (BDH Chemicals Ltd, Poole, Dorset, UK) and other reagents were Analar grade. PERMEABILITY EXPERIMENTS On day 1, the in vitro permeability of [3H] water through the membranes, i.e. whole skin or epidermal membranes, was measured using glass diffusion cells (Figure 1). The diffusion cells were made in our own workshops. They exposed a 1.8 cm 2 area of skin surface and their receptor volume was approximately 4.8 mi. All experiments were done at 30 ø ___ IøC. Human skin samples with a permeability constant greater than 1.5 X 10 -3 cm hr-• and rat skin samples with a permeability constant greater than 2.0 X 10 -3 cm hr-• were considered damaged as a result of handling during the experiment and were not used (12). These values were used for both the whole skin and the prepared epidermal membranes. Approximately 80% of the whole skin membranes and 60% of the epidermal membranes were suitable. [3H] water was desorbed from the acceptable skins overnight by soaking in distilled water placed in the donor and re- ceptor chambers. The permeability of the membranes to the test compounds was as- sessed on Day 2 of the experiment. Either [•4C] paraquat solution (applied as a 1 mg/ml aqueous solution of the dichloride salt) or neat toluene was placed in the donor chambers, and either distilled water or 50% v/v aqueous ethanol was placed into the receptor chambers. These donor solutions were left in contact with the membranes until a steady state rate of absorption was measured. Toluene was left in contact for 10 hours and [•4C] paraquat solution for 72 hours. For experiments using radiolabelled penerrants (i.e. [3HI water and [•4C] paraquat), 25lxl samples were taken (Hamilton microsyringes) from the receptor solution during
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)