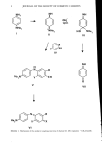
OXIDATIVE HAIR DYEING 3 forms a relatively stable complex with hydroxide ion. This complex is unreactive to- wards the couplers, and dye formation proceeds only through uncomplexed imine II. Since the pH value at which half of the imine is complexed is 8.1, uncomplexed imine is the minority form at pH 8.1. In addition, the complex III collapses irreversibly to give p-benzoquinone monoimine (VII) which will then undergo dye-forming reactions as described previously (3). This mechanism has important consequences for hair dye chemistry. The rate of color formation from the diimine II and the coupler IV is given by d[VI] - kc. [II] v ß [IV]R dt where [II]v represents the concentration of uncomplexed imine and [IV]R represents the concentration of coupler in its reactive form, which is the free base in the case of m-diamine couplers and the phenolate ion in the case of phenolic couplers. The second-order coupling rate constants, k c, are essentially identical for the diimines derived from p-phenylenediamine and its N,N-bis(2-hydroxyethyl) derivative I, re- flecting their similar reactivities. However, the unsubstituted imine, which is only reactive in its conjugate acid form, is converted to the unreactive free base by proton loss as pH is increased (pK a 5.75). Reaction rates for the unsubstituted diamine are thus slower than predicted on the basis of stoichiometric concentrations. It was originally thought that since the disubstituted imine II could not convert to a neutral form by proton loss, its color formation rates would be extremely fast even at high pH. Based on the mechanism of Scheme 1, viz., the formation of III, this predic- tion is invalid. For a given coupler at fixed pH, the rate of dye formation is controlled by the concentration of free diimine II. Since, at pH 9-10, most of the imine is in its unreactive, complexed form (III), the rates of color formation are much slower than expected. However, from a consideration of relative pK values, there is a higher con- centration of the cationic diimine from the disubstituted derivative (pK = 8.1) than of protonated diimine from the parent diimine (pK a = 5.75) at any pH value. Thus, although for the substituted diimine II there is a large rate reduction due to complex formation, the overall color formation rate is still about 2 orders of magnitude faster than that for the parent p-benzoquinone diimine. The mechanism also predicts that under certain conditions much of the disubstituted diimine will be hydrolyzed to p-benzoquinone monoimine and dyes will then be formed from the monoimine. Minimum hydrolysis will occur at low pH and high coupler levels. Since the pH of oxidation dyeing is fixed at around 9-10, where hydrolysis is significant, the reaction course is determined by the coupler to imine ratio. It is fortu- nate in practice that this ratio is high since excess coupler is used to ensure complete reaction of all primary intermediates to the appropriate dyes. Generally, therefore, hy- drolysis will not be a competing process during a typical dyeing procedure and the substituted p-diamine will be converted mainly into the corresponding indo dye VI. As was pointed out in a recent article (4), in actual practice dyes are formed sequentially rather than concurrently due to differences in oxidation potential of the various primary intermediates. Thus, in a mixture of p-phenylenediamine and its bis-hydroxyethyl ana- logue, dyes based on the substituted analogue will be formed first. These dyes will also be formed preferentially if the supply of coupler is limited.
4 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS DYE SPECTRA Spectral data for a number of N-substituted 2-amino indamines VIII (X = NH) and indoanilines VIII (X = O) are shown in Table I. For comparison, literature values for the corresponding N,N-dimethyl and the unsubstituted analogues are also given. It is clear that di-substitution of the amino group on the benzenoid ring gives a shift of 70-90 nm to higher wavelength to the spectral maximum of the monocationic form of these dyes. N-methyl and N-2-hydroxyethyl substituents have similar effects. In addi- tion, there is also a significant bathochromic shift to the spectrum of the neutral form of the indamines. In contrast, N,N-disubstitution has almost no effect on the spectrum of the indamine dication, showing that the second protonation occurs on the 4'-amino group and effectively removes any influence of this group from the spectrum. The strong bathochromic shift is of considerable importance in oxidation dye chem- istry. The blue colors produced allow for the use of couplers which only give magenta with p-phenylenediamine. Thus, the ash, dark brown, and black shades which require use of m-diamine couplers if formulated with p-phenylenediamine as the only primary intermediate can be formulated with a coupler such as 1-naphthol. The blue indo-ani- line dye produced is also of greater stability than the conventional amino-indamines. Furthermore, even if m-diamines are used, the effect of the bis-hydroxyethyl group on 'the second pK• of the indamine is beneficial. This pK• has a value of 3.6 -+ 0.15 for indamines derived from p-phenylenediamine. In the current work we have determined a pK• value for N-dimethylamino indamines of 3.7 + 0.1 and for N,N-bis-hydroxyeth- ylamino of 2.4 _+ 0.3. It is apparent, therefore, that the bis-hydroxyethyl substituent has a special effect on this pKa, making it more difficult to protonate the amino group. The overall effect, therefore, is that the stable indamine monocation is the major ionic form over a wider pH range and, in particular, is still the major form in the pH range 3-5, which is typical of the pH in hair and of acid perspiration. Color changes are Table I Spectral Data [h.m• x (log E)] and pK a Values for Indo Dyes (VIII) •N• •1 (VIII) R R' R • X Dication pK a Monocation pK a Neutral CH2CH2OH CH2CH2OH H Nit 425 (3.69) 2.4 633 (4.38) 11.3 535 (4.02) CH2CH2OH CH2CH2OH CH 3 NH 460 (3.62) 2.6 636 (4.25) 11.1 523 (3.92) CH2CH2OH CH2CH2OH C1 NH 430 (3.63) 2.7 660 (4.27) 10.7 530 (3.98) CH2CH2OH CH2CH2OH OCH 3 NH 415 (3.72) 2.0 626 (4.21) 10.7 505 (3.97) CH 3 CH 3 CH 3 NH 447 (3.80) 3.7 640 (4.35) 10.9 468 (3.97) H il CH 3 NH 455 (3.78) 3.6 549 (4.09) 10.6 464 (3.85) CH2CH2OH CH=CH2OH H O .... 580 (4.16) CH2CH2OH CH2CH2OH CH 3 O -- -- -- 568 (3.92) CH 3 CH 3 CH 3 O -- -- -- 550 (3.90) H H CH 3 O -- -- -- 492 (3.96) H CH2CH2OH CH 3 NH 440 (3.74) 2.7 614 (4.19) 11.0 480 (3.92) H CH 3 CH 3 NH 434 (3.89) 3.8 620 (4.32) 10.8 474 (3.98)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)