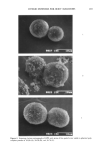
j. Soc. Cosmet. Chem., 41, 155-171 (May/June 1990) Micelies, microemulsions, liquid crystals, and the structure of stratum corneum lipids STIG E. FRIBERG, Department of Chemistry, Clarkson University, Potsdam, NY 13699-5810. Received Dec. 1989 Synopsis Surfactants form amphiphilic association structures such as micelies, vesicles, microemulsions, and lyo- tropic liquid crystals when combined with each other and with water. These association structures play a decisive role in cosmetics, covering all the steps from the preparation of cosmetic formulation over the intermediate stages after the application on the skin to the final interaction with the stratum corneum lipids, which themselves are in the form of an amphiphilic association. INTRODUCTION Surfactants are well known in cosmetic science they have been applied as stabilizing agents in cleaning vehicles as well as in beautifying formulations since the beginning of such efforts (1-3). So far their effect has been considered mainly from the point of view of stabilizing an interface in an emulsion or a foam, or solubilizing small amounts of an oil compound into an aqueous miceIlar solution. However, this limited view does not reflect the essential function of the surfactants and leaves an incomplete opinion about their true action. In this article the aim is toward a complementary description of their role, pointing out some specific structural effects, which are of decisive importance in their application. The treatment is organized with an initial, brief description of the amphiphilic associa- tion structures. The second section outlines the role of these structural organizations in different formulations, the third one deals briefly with the fate of formulations on the skin, and the final one reflects the stratum corneum lipid structure against the final residue of cosmetic components on the skin. AMPHIPHILIC ASSOCIATION STRUCTURES These structures are of two principle kinds. In solutions micelies and vesicles are formed as separate microdroplets that move freely in the liquid. The total appearance is, hence, an isotropic and transparent liquid. On the other hand, the lyotropic liquid crystals are 155
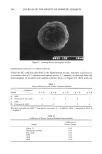
156 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS precipitated out from solutions, forming separate phases with gel-like properties. The relations between these structures are easy to comprehend if their geometrical back- ground is clarified. Ninham and Israelachvili (4) have given a simple rule to obtain this background. One has directly from geometrical considerations of a sphere, a cylinder, and a lamellae R = v/a/ [ 1] in which v is the real volume of the hydrocarbon chain, a is the cross-sectional area of the surfactant in the structure in question, and l is the approximate length of the surfactant hydrocarbon chain. A physical interpretation of this formula is useful to illustrate its implications. The value of a in Equation 1 is not the geometrical cross-section of the polar part of the surfactant per se but the area including one half of the distance to the closest neighbor (Figure 1). In this manner the ratio R is the ratio between the hydro- carbon chain real volume and a volume calculated by multiplying the area a by the length 1. It is easily realized that when these two volumes are equal, the molecules tend to pack in a lameliar arrangement. However, this packing is retained for all R values in the range 1-0.5. At R = 0.5, a new packing is obtained: a cylinder. Table I shows spheres to be stable for R values below 1/3. The structures in Table I appear in typical cosmetic formulations as a natural conse- quence of the geometry of the compounds involved and they can be predicted with some accuracy. So, for example, will an ionic surfactant with its large a (from electric repul- sion) and, hence, low R values, and small spherical micelies, be useful for transparent aqueous lotions. For an emulsion or a cream, on the other hand, parallel packing is required for stability, and a combination of the ionic surfactant with a long-chain al- cohol gives the appropriately large R value. Hence, the structures in Figure 2, which may, at first, appear mainly of academic Figure 1. The expression R = v/al is the quotient between the real volume of the hydrocarbon chain (v) and the volume of the area a times the length of the molecule 1.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)