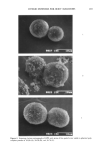
AMPHIPHILIC ASSOCIATION STRUCTURES 159 polar group is small and the lameIlar liquid crystal is formed directly from the aqueous solution. The aqueous solubility is now extremely small ( 1%), and the lameliar liquid crystal is in equilibrium with a very dilute aqueous solution. This fact is used to prepare creamy dispersions of the liquid crystal and also to prepare vesicular solutions. In the following sections, emulsions, microemulsions, and vesicles are briefly described, emphasizing situations when surfactant association structures are important for special properties of the system. EMULSIONS Emulsions were originally considered as dispersions of one liquid in another in the form of large droplets (micron size) (5). The stability of such an emulsion is decided by the surface properties of the droplets and the colloid stability of the thin liquid films formed when droplets fiocculate (6). However, a large number of cosmetic emulsions (3) contain more than two liquids they may, in addition, contain another liquid, a solid substance or a lameliar liquid crystal. The first and last cases are of interest for this article because they illustrate the decisive influence that surfactant association structures can have on emulsion properties. THREE LIQUIDS Emulsions containing three liquids stabilized by the common ethylene oxide adducts are characterized by a very small average droplet size in spite of only a minute energy required for the emulsification process (7,8). The emulsification of an O/W system takes place at elevated temperatures (HLB-temperature, =60 C ø) where the third liquid (the surfactant phase) appears (Figure 4A). The interfacial tension between the three liquids is at minimum at the HLB-temperature and emulsification is facilitated. After emulsification, the system is cooled fast to room temperature. The surfactant phase region has now been moved to the water corner, and the emulsion becomes a traditional one of two liquid phases (Figure 4B). The "move" of the surfactant phase means a rather tumultuous process in the system, the surfactant phase being split into two phases and its components transferred to the oil and water liquids. These interfacial transfers fur- ther divide the droplets into smaller ones. TWO LIQUIDS PLUS A LIQUID CRYSTAL When the third phase is a liquid crystal (Figure 5) (9,10), two properties are interesting from the formulation point of view. At first it should be observed that the vehicle that stabilizes the emulsion (liquid crystal, Figure 5) is now composed not only of the sur- factant: the water and the hydrocarbon are also included into it. As a matter of fact, in the figure, they are the major components. In the example the liquid crystal has a composition of water 59.4%, oil 24.4%, with the surfactant present only to 16.2% (by weight). Hence, the stabilizing body consists of 83.8% of water and oil. This vehicle exists in the emulsion together with the oil phase and aqueous phase and can be sepa- rated from them by centrifugation. Instead of the common two layers, when a simple emulsion is separated, now three layers are found: oil, water, and liquid crystal.
160 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Water HYDROCARBON Oil (B) " SurfactanphaseOil•HYDRxphase•" •_ WATER (A) Aqueous phase WATER Cx (EO) y Figure 4. At the HLB temperature (A) the isotropic liquid (surfactant phase) is a conspicuous part of the diagram. It disappears when the temperature is reduced by 30øC. Cx(EO)y = CxH2x +•[(CH2)20]yOH The third layer, the liquid crystal, appears as soon as the surfactant concentration ex- ceeds the value X in Figure 5 for an emulsion with an oil/water weight ratio of 1:1. Hence, in the surfactant concentration range X-Y (Figure 5), two kinds of stabilizers act: the surfactant molecules dissolved in the water and in the oil, and the liquid crystalline phase. The surfactant concentration in the aqueous phase is 2.5% by weight (P, Figure 5) and in the oil phase 5.8% (Q, Figure 5). These concentrations do not change when the total emulsifier concentration is increased from 4.0 to 13.5% by weight (X-Y, Figure 5). The amount of liquid crystal varies strongly, on the other hand, when the surfactant concentration is increased from 4.0 (X, Figure 5) to 13.5% by weight (Y, Figure 5). The latter composition results in a system with 72% liquid crystal plus 28% oil phase, with 5.8% emulsifier in it. A fourfold increase in emulsifier concentration results in a 24-fold stabilizer increase! Aside from the obvious role of stabilizer, the liquid crystal has recently also been used as a preparatory tool (11,12). The liquid crystal forms spontaneously from the components (Figure 5) and will distribute the oil into small droplets when stirred into water. MICROEMULSIONS The microemulsions are fundamentally different from emulsions, Table II (13). Their small droplets (0.0015-0.15 •m) make them transparent. These droplets form sponta- neously to stir a system down to dimensions in this range is obviously impossible.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)