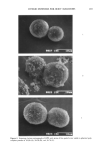
176 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS HOa$O CH 3 4-methylumbelliferyl surf ate aryl sulf HOOC HI :3 0 0 0 CH 3 4-methylumbelliferyl glucuronide curonidase HO CH3 4-methylumbelliferol Figure 3. Hydrolysis of 4-MUS and 4-MUG by aryl sulfatase and beta-glucuronidase. umbelliferyl glucuronide, 4-methylumbelliferyl sulfate, 4-methylumbelliferone, and D-saccharic acid-A-lactone were purchased from Sigma Chemical Co. Sodium hexame- taphosphate (SPORIX) was obtained from International Sourcing. Apocrine secretion was obtained from Ivy Research Labs (Philadelphia, PA). The fluid was collected according to the method described by Labows et al. (10). Eleven subjects were used. The axillary area was shaved, washed with a 0.1% solution of Triton X-100, rinsed with water, blotted dry, then rinsed with hexane. An intradermal injection of 0.1 ml of 1:2000 adrenaline was performed to stimulate the apocrine glands. Micropi- pettes (10 •1) were placed directly against the skin to collect the droplets of apocrine secretion. Samples were frozen until ready for use. Samples were eluted from the capil- lary tubes with 0.1 M Tris buffer, pH 7.0, for use in odor-generation studies. Lipo- philic diphtheroid cultures were provided by Dr. John Labows at Monell Chemical Senses Center (Philadelphia, PA) they were cultures of strains originally isolated from human axillae at Monell. Zinc glycinate was prepared by the method of Dubsky and Rabas (16): 52.5 g (0.6 mol) glycine and 20.35 g (0.25 mol) ZnO were stirred in 300 g water at 75 C until a clear
AXILLARY MALODOR 177 solution was obtained, and after cooling the product was precipitated with 300 g eth- anol. The white crystals were washed three times with absolute ethanol and dried in vacuo. Zinc as determined by atomic absorption was 27% (theoretical, 30%), and gly- cine by Kjeldahl was 68% (theoretical 70%). Solubility of zinc glycinate (Zn-GLY) in water at 20øC and pH 7.2 was found from zinc concentration of a saturated solution to be 5 g/100 mi. METHODS PILOT STUDY A double-blind screening study was designed to detect the enzymes beta-G and AS in axillary secretions. Swabs were taken from the axillae of twenty men, classified by professional odor evaluators (Hilltop Laboratories, Cincinnati, OH) as 10 "high-odor formers" and 10 "low-odor formers." Trypticase soy agar plates were prepared con- taining either 4-MUS (substrate for aryl sulfatase) or 4-MUG (substrate for beta-glu- curonidase) at a concentration of 25 ppm. Elutions of the swabs were plated on both substrates, incubated for 24 hrs, and the fluorescence of the plates was estimated visually. SEMIQUANTITATIVE ASSAYS OF BETA-GLUCURONIDASE AND ARYL SULFATASE The enzyme assays measure conversion at 37øC of the non-fluorescent substrates 4-MUG (for beta-G) and 4-MUS (for AS) to fluorescent product, 4-MU (Figure 2). The assay mixtures contained: For beta-G: For AS: 0.65 ml water 0.5 ml 0.1 M Tris buffer, pH 7 0.25 ml 0.01 mg/ml 4-MUG in water 0.1 ml 0.001 mg/ml beta-G in Tris (0 mg/ml beta-G/Tris as control) 1 ml water 1.7 ml 0.1 M Tris buffer, pH 7 0.2 ml 0.5 mg/ml 4-MUS in water 0.1 ml 0.01 mg/ml AS in Tris (0 mg/ml AS/Tris as control) For semiquantitative studies, fluorescence was detected by long-wave UV lamp (Black- Light Eastern Corporation, Model XX 15). The effects of inhibitors on the enzymatic reactions were assessed by substituting aqueous solutions of test inhibitor, at 0.1 mM to 100 mM, for the water. To rule out quenching of the fluorescence reaction by the inhibitors, separate tests were performed comparing the fluorescence from 4-MU at the maximum concentration expected in the enzyme reaction mixtures with either inhibitor solution or water. QUANTITATIVE ASSAYS OF BETA-G AND AS Several inhibitors of each, beta-G and AS, were assayed by quantitative fluorimetry. Studies were carried out with a Perkin-Elmer model MPF-3 with an Osram XBO high pressure Xenon lamp, using quartz curettes only. Prior to the performance of quantita- tive assays it was necessary to determine absorption and emission maxima of the fluores-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)