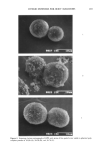
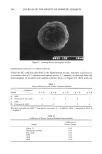
j. Soc. Cosmet. Chem., 41, 187-195 (May/June 1990) Chronic ultraviolet radiation-induced skin tumors and associated changes in basal epidermal ornithine decarboxylase activity GREGG. HILLEBRAND, MARCIA S. WINSLOW, DAVE A. HEITMEYER, and DONALD L. BISSETT, The Procter & Gamble Company, Miami Valley Laboratories, Cincinnati, OH 45239-8707. Received April 13, 1990. Synopsis The association between ultraviolet radiation (UVR)-induced skin neoplasia and the basal levels of the epidermal enzyme ornithine decarboxylase (ODC) was studied in Skh:HR-1 hairless mice. Groups of mice were chronically exposed to UVR (15 weeks), then treated with photoprotective agents (12-20 weeks) with or without continued UVR exposure. After treatment, total skin papilloma/carcinoma (tumor) area per mouse and epidermal ODC activity (in the treated and UVR-exposed but non-tumor-involved dorsal skin) were measured in each group. Mice with the largest average tumor area had substantially elevated basal ODC activities (•350-fold) relative to non-irradiated control mice, which lacked tumors. Groups of mice treated with photoprotective agents showed intermediate levels of skin tumors and correspondingly inter- mediate epidermal ODC activities. Basal epidermal ODC activity was also measured in lifetime sun-exposed and unexposed skin of four healthy human volunteers with no history of skin cancer. No significant relationship was observed between human epidermal ODC activity and prior history of solar exposure in these individuals. These human data are consistent with recent hairless mouse studies [Hillebrand, G. G., Winslow, M. S., Benzinger, M. A., Heirmeyer, D. A., and Bissett, D. L., Cancer Res., 50, 1580-1584 (1990)] showing that chronically irradiated mice lacking visible tumors had normal levels of epidermal ODC activity. The results support the idea that elevated levels of epidermal ODC activity may be specifically indicative of chronic UVR-in- duced neoplastic growth. INTRODUCTION The visible appearance of skin, especially facial skin, can be very dependent on an individual's past history of solar radiation exposure. For example, chronic solar radia- tion exposure can lead to damage of the underlying dermal connective tissue (1-4), manifested as accelerated visible skin aging in the form of wrinkles (5-8). In more severe cases, chronic photodamage can progress to the most common forms of skin cancer, i.e., basal and squamous cell carcinoma (9). The diagnosis and development of therapy for photodamaged skin is facilitated by the employment of biochemical markers 187
188 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS of chronic skin photodamage and/or neoplasia. The epidermal enzyme ornithine decar- boxylase (ODC) is potentially such a marker. ODC (EC 4.1.1.17) is the rate-limiting enzyme in the biosynthetic pathway of poly- amines, macromolecules essential for cell proliferation (10). In normal epidermis, basal ODC activity is relatively low. Acute exposure of skin to ultraviolet radiation (UVR) or chemical tumor promoters can cause transient induction of epidermal ODC activity. Repeated induction of epidermal ODC activity is thought to be essential, but not sufficient, for skin tumor promotion, the second stage of skin carcinogenesis (11,12). The association of ODC with neoplasia has led to the wide use of acute ODC induction as a short-term biochemical marker for long-term skin tumor promotion by chemicals and UVR in tissue culture models (13-15) and animal models (11,16-23). Recently, Hillebrand et al. (18) showed that chronic UVB radiation exposure leads to greatly elevated levels of basal epidermal ODC activity in hairless mice. Elevated levels of ODC activity have also been associated with hyperplastic skin disorders (17,24-26) and ma- lignant tumors (10,17,27) including squamous cell carcinomas. These studies support the potential of ODC as a biochemical marker for chronic skin photodamage and neo- plastic growth. The purpose of the present study was to determine if visible skin photodamage is asso- ciated with increased basal levels of epidermal ODC activity. Also, we wanted to know if photoprotective agents such as sunscreens, antioxidants, and iron chelators that in- hibit skin photodamage (28-30) might also affect basal ODC activity. While sun- screens and antioxidants protect skin from photodamage by direct UVR absorption (36) and scavenging of oxygen radicals (29), respectively, the photoprotective mechanism of iron chelators is less well known. Iron chelators are believed to be photoprotective by sequestering iron, thereby preventing the iron-catalyzed formation of damaging oxygen species. The following is the presumed mechanism: UVR damages blood vessels in the skin, resulting in increased permeability to iron-binding proteins such as transferrin. Iron then accumulates in the UVR-exposed skin. It is known that iron can act as a catalyst in the generation of damaging oxygen radicals (31), and evidence strongly suggests that oxygen radicals are involved in skin photodamage (32). Iron chelation may prevent generation of the damaging oxygen radicals. EXPERIMENTAL MATERIALS L-[1-14C]Ornithine hydrochloride (specific activity = 51.6 mCi/mmol) was purchased from New England Nuclear (Boston, MA). tx-Tocopherol was purchased from Sigma Chemical Co. (St. Louis, MO) and applied topically as a solution in 100% ethanol. 2,2'-Dipyridylamine was purchased from Aldrich Chemical Co. (Milwaukee, WI) and applied topically as a solution in simple vehicle (water:ethanol:propylene glycol, 1:2:1, v:v:v). Suncreen (containing p-methoxy cinnamate and 4-p-butyl-4'-methoxydibenzoyl methane) had a sun protection factor (SPF) of 8. All other chemicals were of analytical grade.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)