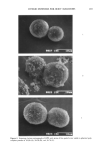
AMPHIPHILIC ASSOCIATION STRUCTURES 161 OIL Q Y P ß 0 :-:":'.i. ' Liquid Crystal WATER SURFACTANT Figure 5. In many emulsion systems a liquid crystal appears at moderate emulsifier concentrations. The microemulsions may be oil (W/O) or water continuous (O/W) or, for some systems, even bicontinuous. They may be stabilized by a single nonionic surfactant (14, 15), a single anionic hydrophobic surfactant such as Aerosol OT (16, 17), or by a combination of an ionic water-soluble surfactant and a cosurfactant (e.g., a medium-chain-length alcohol) (18, 19). Aerosol OT (sodium di-(2ethyl hexyl)sulfosuccinate) stabilizes W/O microemulsions with a comparatively high ratio of surfactant to water, and is used for Table II Properties of Emulsions and Microemulsions Property Emulsions Microemulsions Appearance Turbid Transparent Droplet radius --0.15-100 IJtm 0.0015-0.15 IJtm Formation Stirring, etc. Spontaneous Thermodynamic stability Unstable Stable (exceptional cases unstable)
162 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table III Nonionic Surfactants for Microemulsions Hydrocarbon/ microemulsion W/O O/W Aliphatic C 12EO 3 C 12EO3 Aromatic C 12EO3 C 12EO 10-12 special purposes. The nonionic surfactants for microemulsions depend on the kind of hydrocarbon used Table III gives an indication of suitable structures. The combination of an ionic surfactant and cosurfactant is used for microemulsions with the phase diagram in Figure 2 as a basis. W/O microemulsions are formulated by addition of hydrocarbon to the alcohol solution and constitute a continuous region from that area (Figure 6) (18). The O/W microemulsion regions are extensions from the miceliar solutions in the water corners, according to Figure 7 (20). The oil is solubilized to some extent into the lameIlar liquid crystal, and the final diagram looks approximately as in Figure 8. VESICLES The lameIlar liquid crystal would require an R value of 1.0, but the lameIlar structure is the only one geometrically possible for R values in the whole range 1.0-0.5 (Table I). Hence, compositions with R values close to 0.5 do not have a structure at the lowest possible free energy, and modifications to enhance the volume of the hydrocarbon chain (Equation 1) would be favored energetically. This fact has been used to enhance the W/O MICROEM ULS!ONS C=OH + x % CeH• C•OH HtO HtO + x%CeHe KOL+ x%CeH e Figure 6. The W/O microemulsions emanate in a continuous region from the alcohol solution in the system without hydrocarbon.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)