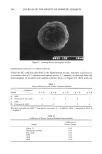
UV-INDUCED SKIN TUMORS 193 "-' 5.0 E2.0 o• 1.0 m E 1 0.0 0.0 12 0 r=0.8 5 10 40 b 1 90 i I I I 0.5 1.0 1.5 2.0 Average Tumor Area/Mouse (cm 2) Figure 2. Correlation between basal epidermal ODC activity and total tumor area in hairless mice (plot of data in Table I). Groups of mice (15-20) were exposed to UVR for 15 weeks, then treated with photopro- tective product for 12 or 20 weeks in the presence (continue UVR) or absence (stop UVR) of continued irradiation. At the end of the treatment period, five mice from each group were killed and basal epidermal ODC activity determined in the non-tumor-involved, UVR-exposed treated dorsal skin. The average total tumor area per mouse was determined from all the animals in each group. Average standard error for ODC determinations was _ 38% and for tumor areas, _ 25%. Data point descriptions are in Table I. in skin sites with extensive photodamage (outer arm, dorsal neck, and preauricular area). Therefore, in the four subjects examined who lacked visible signs of skin cancer, the basal level of epidermal ODC activity did not consistently reflect the extent of prior solar exposure. These results are reminiscent of previous animal studies. No significant increase in basal epidermal ODC activity is observed in chronically irradiated hairless mice lacking visible signs of neoplasia (18). Basal epidermal ODC activity in UVR-ex- posed non-involved skin increased only at the time tumors first appeared. In summary, basal levels of epidermal ODC activity correlate well with the degree of Table II Human Basal Epidermal ODC Activity in Chronically Solar-Exposed and Unexposed Skin Inner Upper Outer Dorsal Preauricular Buttock arm back arm neck area Subject ! 411.6 (0)* 669.3 (0) 16.3 (1.5) 10.1 (3) 24.5 (5) 64.3 (3.5) Subject 2 325.0 (0) 387.8 (0) 149.7 (1) 25.4 (3) 12.0 (3.5) 20.2 (4) Subject 3 10.5 (0) 89.1 (0) 45.3 (2) 53.4 (4) 294.6 (5) 26.5 (4.5) Subject 4 40.6 (0.5) 12.0 (0) 34.3 (2.5) 169.1 (2) 207.6 (3.5) 137.5 (1.5) Average 197 (0.1) 290 (0) 61.4 (1.75) 64.5 (3) 134.6 (4.25) 62.1 (3.75) * ODC activity expressed as pmols CO2/h/mg protein. Numbers in parenthesis represent skin photo- damage grades on a 0-5 scale: 0, no photodamage 5, severe photodamage.
194 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS visible skin photodamage assessed by total tumor area in hairless mice. Topically ap- plied agents that protect skin from chronic UVR-induced damage also inhibit elevation of basal ODC activity. In humans with no history of skin cancer, skin sites chronically exposed to solar radiation did not show consistently higher basal epidermal ODC activi- ties. These data suggest that constitutively elevated levels of epidermal ODC in chroni- cally UVR-exposed skin are specifically associated with signs of neoplasia. Photo- damaged skin not exhibiting signs of neoplasia has normal levels of epidermal ODC activity. It would be interesting to determine human ODC activity in normal-ap- pearing skin adjacent to solar-induced premalignant and malignant skin cancers where elevated basal ODC activity would be expected. ACKNOWLEDGMENTS We gratefully acknowledge the expertise of Daniel P. Hannon in grading the human skin sites for photodamage. We also thank Hill Top Research (Cincinnati, OH) for their capable assistance in conducting the human experiments. REFERENCES (14) (15) (1) L. H. Kligman, F. J. Akin, and A.M. Kligman, Prevention of ultraviolet damage to the dermis of hairless mice by sunscreens, J. Invest. Dermatol., 78, 181-189 (1982). (2) W. M. Sams, J. G. Smith, and P. G. Burk, The experimental production ofelastosis with ultraviolet light, J. Invest. Dermatol., 43, 467-471 (1964). (3) J. M. Knox, E. G. Cockerrel, and R. G. Freeman, Etiological factors and premature aging,J. Am. Med. Assoc., 179, 630-636 (1962). (4) A. M. Kligman, Early destructive effect of sunlight on human skin, J. Am. Med. Assoc., 210, 2377-2380 (1969). (5) R. M. Lavker, Structural alteration in exposed and unexposed aged skin, J. Invest. Dermatol., 73, 59-66 (1979). (6) D. L. Bissett, D. P. Hannon, and T. V. Orr, An animal model of solar-aged skin: Histological, physical, and visible changes in UV-irradiated hairless mouse skin, Photochem. Photobiol., 46, 367-378 (1987). (7) L. H. Kligman and A.M. Kligman, "Cutaneous Photoaging by Ultraviolet Radiation," in Models in Dermatology, H. I. Maibach and N.J. Lowe, Eds. (Karger, Basel, 1985), Vol. I, pp. 59-68. (8) K. J. Johnston, A. I. Oikarinen, N.J. Lowe, and J. Uitto, "Ultraviolet-Induced Connective Tissue Changes in the Skin: Models for Actinic Damage and Cutaneous Aging," in Models in Dermatology, H. I. Maibach and N.J. Lowe, Eds. (Karger, Basel, 1985), Vol. I, pp. 69-76. (9) J. Scotto, T. R. Fears, and J. F. Fraumeni, Incidence of Non-Melanoma Skin Cancer in the United States, Washington, DC: US Department of Health and Human Services, 1981: (NIH)82-2433. (10) A. E. Pegg, Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy, Cancer Res., 48, 759-774 (1988). (11) T. G. O'Brien, The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis, Cancer Res., 36, 2644-2653 (1976). (12) T. J. Slaga, Multistage skin carcinogenesis: A useful model for the study of the chemoprevention of cancer, Acta Pharmacol. Toxicol., 55 (Suppl. 2), 107-124 (1984). (13) H. J. Niggli and R. R6thlisberger, Cyclobutane-type pyrimidine photodimer formation and induc- tion of ornithine decarboxylase in human skin fibroblasts after UV irradiation, J. Invest. Dermatol., 91, 579-584 (1988). U. Lichti, G. T. Bowden, E. Patterson, T. Ben, and S. H. Yuspa, Germicidal ultraviolet light induces ornithine decarboxylase in mouse epidermal cells and modifies the induction caused by phorbol ester tumor promoters, Photochem. Photobiol., 32, 177-181 (1980). U. Lichti, S. H. Yuspa, and H. Hennings, "Ornithine and S-Adenosylmethionine Decarboxylase in
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)