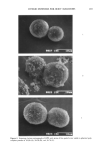
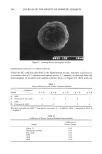
206 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS well resolved. Other minor peaks are probably due to volatile contaminants deriving from the original acid reagent. The coefficient of variation (cv %) of GC peak areas for five consecutive standard acid solution acquisitions was 3.2% for isovaleric acid and 4.0% for caproic acid. A chromatogram obtained by adding standard acid solution to talc is shown in Figure 6B. The GC profile looks more or less identical to Figure 6A, which means that talc has hardly any effect in suppressing short-chain fatty acids. On the other hand, as can be seen from Figure 6C, zinc oxide has considerably suppressed the acids. The OSR values of the candidates are summarized in Table III. As expected, fine-particle zinc oxide suppressed the acids most efficiently, giving OSR values of over 90%. The chemical reaction to convert odorous short-chain fatty acids into odorless metallic salts as shown below appears to be proceeding. 2RCOOH + ZnO-• (RCOO)2Zn q- H20 R: alkyl group odorous odorless This reaction has already been confirmed by Fourier transform infrared spectrophotom- etry (FT-IR) (3,6). Talc and spherical polyethylene powder had no effect at all in sup- pressing the acids. Benzalkonium chloride, an antimicrobial agent, had no effect in suppressing isovaleric acid and very litle effect for caproic acid. Aluminum chlorohy- drate gave OSR values of 16.5% for isovaleric acid and 40.6% for caproic acid. The quenching mechanism, possibly physical adsorption or formation of certain complexes, is not yet understood, although the values seem high when one considers the strong acidity of aluminum chlorohydrate. The hybrid powders were sampled in two different ways, 10 mg like all the other powders, and also to contain 10 mg as zinc oxide or aluminum chlorohydrate (i.e., 33.3 mg sampling). For HPZ sampled to contain 10 mg as zinc oxide, OSR values of 90.6% and 91.7% were obtained. This is reasonable, since spherical polyethylene powder possesses no ability to suppress short-chain fatty acids, indicating that zinc oxide's quenching power is retained even in its hybridized form. The OSR values obviously decrease for 10-mg sampling due to the use of less zinc oxide. HPA sampled to contain 10 mg as aluminum chlorohydrate gave OSR values of 21.8% and 49.7%, which were somewhat higher than expected compared to those obtained from aluminum chlorohydrate alone. This indicates the enhancement of alu- minum chlorohydrate's quenching power upon hybridization. Table III OSR Values of Various Compounds OSR (%) of OSR (%) of Sample isovaleric acid caproic acid Zinc oxide 91.1 91.8 ACH 16.5 40.6 Talc 0 0 Polyethylene powder 0 0 Benzalkonium chloride 0 4.4 HPZ (10 mg) 54.2 52.8 HPZ (10 mg as ZnO) 90.6 91.7 HPA (10 mg) 9.6 21.9 HPA (10 mg as ACH) 21.8 49.7
HYBRID POWDERS FOR BODY MALODORS 207 CONCLUSIONS Hybridization of spherical polyethylene powder and fine-particle zinc oxide or fine-par- ticle aluminum chlorohydrate resulted in hybrid powders that possessed the advantages of both the core and the outer layer powders. The texture of fine-particle zinc oxide and fine-particle aluminum chlorohydrate im- proved drastically when hybridized with spherical polyethylene powder. The results obtained from the direct evaluation method and instrumental evaluation method were in agreement. The quenching power of zinc oxide was completely retained and, in the case of alu- minum chlorohydrate, was even enhanced when hybridized. In this study we have emphasized the effectiveness of hybrid powders as quenching actives to eliminate body malodors. Numerous variations of hybrid powders can be achieved by changing the core powder and the outer layer powder. This hybridization technology we believe will be applied widely in the field of cosmetics and pharmaceu- ticals in the future. REFERENCES (1) T. Nakane, Y. Yahata, K. Yoshida, and T. Nanba, Hybrid fine powder, Boundary, 1, 28-30 (1985). (2) T. Nakane, T. Nanba, and K. Tomira, Development and functions of hybrid powders by mechano- chemical methods, 2 6th S C CJ C onj•rence Preprints, 17-18 (1989). (3) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, O. Nakata, and Y. Fujiyama, Elucidating body malodour to develop a novel body odour quencher, 15th 1FSCC International Congress Preprints, 3, 529-562 (1988). (4) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, O. Nakata, and Y. Fujiyama, Elucidating body malodour to develop a novel body odour quencher, J. Soc. Cosmet. Chem. Japan, 23, 217-224 (1989). (5) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, and O. Nakata, Elucidation of chemical compounds responsible for foot malodour, Brit. J. Dermatol., 122, 771-776 (1990). (6) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, and O. Nakata, Development of a novel hybrid powder formulated to quench body odor, J. Soc. Cosmet. Chem., 40, 335-346 (1989). (7) Y. Fujiyama and F. Suzuki, Cosmetics and powder technology, Funtai Ko-gaku-kaishi, 9, 565-573 (1984).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)