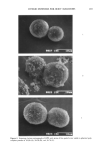
J. Soc. Cosmet. Chem., 41, 209-212 (May/June 1990) A note on the permanent setting of human hair MAX FEUGHELMAN, School of Fibre Science and Technology, The University of New South Wales, Kensington, N.S.W. 2033, Australia. Received July 30, 1990. Synopsis In the standard permanent setting procedure for human hair, ammonium thioglycollate is applied to the curled hair. Disulfide bonds are converted to sulfhydril groups to enable the protein structure of the hair fibers to relax mechanically by the mechanism of sulfhydril disulfide interchange. After relaxation, the sulfhydril groups are reoxidized (neutralized) to reform the disulfide bonds, thus stabilizing the curled conformation. If the mechanical relaxation is carried out at an elevated temperature, the relaxation can occur with a much lower coversion of disulfide to sulfhydril groups. If at these elevated temperatures, the density of sulfhydril groups formed is low, returning the hair fibers to room temperature is sufficient to stabilize the curl. This eliminates the need for oxidation of the sulfhydril groups back to disulfides and results in major benefits of time saved and fiber degradation as observed by appearance and feel. Other benefits are also noted for the application of this technique to the permanent waving procedure. In the permanent waving of human hair the overall procedural steps normally applied are as follows: 1. The hair fibers are shaped into the proposed, i.e., wrapped on a normal perm-roller. A reducing agent that penetrates into the hair is applied. 2. The reducing agent results in the cleavage of the covalently bonded disulfides of the cystine bonds, which cross link the protein chains forming the hair fibers. The cleavage results in the formation of two sulfhydril groups from every disulfide group reduced. 3. With the cleavage of the cystine bonds, the protein chains of the keratin structure of the hair fibers are able to rearrange to reduce or remove the forces present in the structure (which would tend to return each fiber to its original configuration on being released from the perm-roller). 4. The arrangement of the protein chains, which has occured in the curled fibers being set, is stabilized by reforming the cystine bonds through the use of a suitable oxi- dizing agent (the neutralizer). It is well recognized that the time of application of the waving solution consisting of a reducing agent such as ammonium thioglycollate, depends on the pH and temperature. What is not emphasized, is that the cleavage of the disulfide bonds is not the prime factor in the rate of rearrangement of the protein chains. The rate is primarily depen- dent on the proportion of ionized sulfhydril groups present, which enables the protein 209
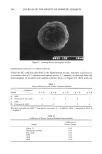
210 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS chains to "flow" by the breakdown and reformation process of sulfhydril-disulfide in- terchange (1,2). This view is supported by measurements obtained on other groups of fibrous keratins, namely wool fibers. It has been shown for wool fibers distorted into a strained configuration that the time rate of protein chain rearrangement is dependent on the reduction of the disulfide bonding (3). If the disulfide bonding is reduced to 42% of the initial content of the fiber with the sulfhydril content fixed by blocking the excess sulfhydrils formed by methylation, the time rate of rearrangement of protein chains is increased by about 40%. This was measured by the relaxation of mechanical forces in the wool fibers in the strained configuration. However, if with the reduction of the disulfide bonding, all the bonds cleaved were converted to sulfhydrils, the large increase in the sulfhydrils present resulted in an increase of the time rate of protein chain rear- rangement by a factor of nearly 20. The above mechanical tests were carried out for wool fibers in distilled water. If the tests were carried out in aqueous solutions over the hydrogen ion concentration range from pH 3.7 to pH 9.7 (2), an increase in the rate of mechanical relaxation and hence of protein chain rearrangement of 1000 for this pH range was obtained. This rate results from the increase of ionized sulfhydril groups and hence from an increase of sulfhydril disulfide interchange. The increase of the rate of mechanical relaxation with increase of temperature also has been shown to correspond to a single reaction process of energy of activation (3) of around 80 kj/g mol. This means that with a rise of temperature for example, from 25øC to 75øC, the rate of mechanical relaxation increases by a factor of 100. The above observations obtained for wool fibers may be considered in relation to steps 1-4 observed in the normal permanent waving procedure for human hair. Steps 1-3 are essentially the steps necessary to make the protein chain of ot-keratin fibers mobile and hence capable of rearrangement to equilibrium with the curled configuration. In the time frame of steps 1-3, the keratin structure acts as a liquid, with a complete relaxation of all forces tending to return the hair fibers to their original configuration. To obtain this structural mobility at or near room temperature, a reduction of some 20%-40% of the disulfide bonds present is normally required in the permanent waving of hair. After sufficient time has elapsed, usually in the range of 10 to 30 minutes, the movement of protein chains is complete. Step 4 is applied to stabilize the rearrange- ment of the protein chains by using a suitable agent to reform the cystine bonds. The protein chains are immobilized by the reformed bonds, and the structural component of the ot-keratin fiber made "liquid" in steps 1-3 is reversed to a mechanically stiff glassy state. The result is that the curled fibers release from the perm-rods in the set configura- tion. When the setting of the hair curl in steps 1-3 is carried out at an elevated temperature, the "liquid" state in the hair fibers necessary for the protein chain rearrangement can be attained with a smaller concentration of sulfhydril groups. That is, a lower cleavage of disulfide bonds is required in steps 1-3, meaning a lower concentration of thioglycol- late can be used. Also, the time to attain sufficient cleavage of disulfide bonds and a complete relaxation of the protein chains into their rearranged state occurs more quickly at elevated temperatures. As a practical example, hair fibers formed into a curl treated with 4% ammonium thioglycollate solution at pH 9.4 at 100øC for about 20 seconds were found to be completely relaxed mechanically, ready for step 4 for the completion of the setting procedure. These fibers were shown from their swelling in concentrated
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)