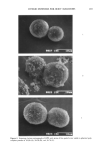
174 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS nature of the odor produced: Where the axillary microbial population is dominated by coryneform bacteria (lipophilic diphtheroids), the acrid odor of delta-16 steroids is ap- parent, whereas, if the axillary population is dominated by such micrococci as Staphylo- coccus epidermidis, the odor of isovaleric acid prevails. Pronounced axillary odor is corre- lated with the occurrence of the coryneform flora (5) it is on the steroid compounds associated with these strains that this research focuses. Many studies have identified axillary steroids and have linked their presence with indigenous bacteria (4- 13) how- ever, the mechanism of bacterial action has remained uncertain (9,12). Since they are not typically water-soluble, steroids are normally transported in body fluids as their water-soluble conjugates with sulfate or glucuronic acid (14). When we began to speculate on the origin of volatile free steroids in the axilla, we hypothesized that apocrine secretion contained the steroids as water-soluble conjugates (1). Conver- sion in vivo to the free steroid generally requires the action of hydrolytic enzymes. It seemed likely that it was the production of these enzymes that represented the contri- bution of bacteria to the generation of underarm oder (Figure 2). We theorized that sterile apocrine sweat would deposit the water-soluble, odorless conjugates onto hair and skin in the axilla, where enzymes secreted by local bacteria would release the vola- tile, odorous, free steroids. The enzymes expected to hydrolyze the steroid esters might be any of several bacterial exoesterases -- for example, beta-glucuronidase (beta-G) and aryl sulfatase (AS). These enzymes can be detected with the synthetic substrates 4-methylumbelliferyl glucuro- nide (4-MUG) and 4-methylumbelliferyl sulfate (4-MUS), respectively, both of which release fluorescent 4-methylumbelliferone (4-MU) upon hydrolysis (Figure 3) (14,15). isovaleric acid CH3 %CH --CH= •COOH 5 ,c•-androst- 16-en-3,[5-ol 5,ct-androst- 16-½n-3-on½ Figure 1. Proposed sources of axillary odor.
AXILLARY MALODOR 17 5 HOOC glucuronate HOOC,• 0 5,{x-androst- 16-ene-3,1•-ol glucuronide androstenol 5,{x-androst-16-ene%sulfate Figure 2. Hydrolysis of steroid conjugates. HOSO 3 • sulfate These fluorogenic substrates are actually models of the steroid glucuronides and sul- fates. We therefore began a series of studies to test Eigen's view, and this paper describes those experiments. MATERIALS , B-glucuronidase from E. coli and aryl sulfatase from Aerobacter aerogenes, 4-methyl-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)