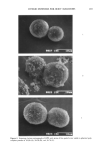
AMPHIPHILIC ASSOCIATION STRUCTURES 157 Table I Relation Between R and Amphiphilic Association Structures R % ¬-« «-1 1 Structure Spherical Cylinder Lamella Inverse cylinder Aqueous or micelie Micelie interest are, in fact, essential to understanding both microemulsions, emulsions, and foams. In addition, the almost identical system of water, soap, and long-chain carbox- ylic acid is decisively relevant to the structure of the stratum corneum lipids. These latter hold the key to the effect of humidity on skin properties and are the essence of cosmetic science. Hence, a closer examination of them is well worthwhile. Adding a long- or medium-chain to an ionic surfactant in an aqueous solution results in three structures forming consecutively. At first, the ionic surfactant forms spherical micelies in the aqueous solution (Figure 2, bottom left). This structure is expected because the polar groups of an ionic surfactant give repulsive forces between them and, as INVERSE MICELLES Long chain Alcohol LAMELLAR LIQUID CRYSTAL water Ionic Surfactant SPHERICAL MICELLES Figure 2. In a system of water, ionic surfactant, and long-chain alcohol three phases are important cosmet- ically. The aqueous solution (lower left) contains spherical micelies, the lameliar liquid crystal is located in the middle, and the alcohol solution (top) contains inverse micelies.
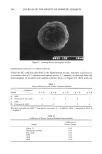
158 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS a consequence, a huge apparent value of a in Equation 1. Hence, spherical micelles are the expected structures in aqueous solutions and they are the ones formed. The spherical micelies are able to solubilize a long-chain alcohol to a maximum of approximately 10%. Addition of more than that amount leads to turbidity the "solubility limit" has been reached and a new phase appears (Figure 2). With a long-chain alcohol and an ionic surfactant, a lameliar packing is actually so favored that a liquid crystal is sepa- rated from a dilute aqueous solution (Figure 2). The structure of this new phase is a result of the combination of the OH group of the alcohol and the charged polar group of the surfactant. The alcohol group has no repul- sion to the charged groups of the surfactant and will fit into the space between them, giving a strong reduction of the average a value in Equation 1. There is no reduction in the v value, and, as a consequence, the R value will exceed the 0.5 limit and a lameliar structure, a liquid crystal, is formed. This structure has two properties of importance. It has a viscous gel-like consistency, which is important for stabilization of many cos- metic formulations. It is also easy to detect in these, because of its birefringence be- tween crossed polarizers. Figure 3 shows a typical microphotograph of a formulation in which a liquid crystal exists in a hand lotion. Alcohol/surfactant ratios in excess of those in the lameliar liquid crystal result in a continued reduction of the a value (Equation 1). The R value in Equation 1 now exceeds 1.0, and inverse micelies are formed in the alcohol solution. The structure of the inverse micelies (Figure 2), with their central water pool surrounded by the surfactant and cosurfactant chains pointing outward, makes them suitable for solubilization of water or aqueous solutions into hydrocarbons. This solubilization is obtained in the microemul- sions, which are discussed later in the article. For other surfactants, such as the double-chain variety (lecithins) or even some single- chain ones such as oligoethyleneglycol-alkyl-ethers, monoglycerides, and similar, the Figure 3. An emulsion with liquid crystals gives an expected microscope picture in normal light (right) but shows shining halos when viewed between crossed polarizers (left).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)