HYBRID POWDERS FOR BODY MALODORS 201 utilized. This analyzer consists of a horizontally placed iron plate on top of which is affixed a double-sided plastic tape to mount the sample. After mounting the sample, an aluminum attachment is placed on top of the sample. After pressing the aluminum attachment at a load of 5-150 g/cm 2, the attachment was moved left and right at a speed of 10 mm/sec. The resulting grinding stress was measured using a strain gauge. A graph was plotted, taking the x axis and y axis as load and grinding stress. The slope of this graph corresponds to the coefficient of kinetic friction. EVALUATION OF HYBRID POWDERS AS QUENCHING ACTIVES Equilibrium headspace gas chromatography (HSGC) was chosen to evaluate various compounds' ability to quench short-chain fatty acid malodors. This methodology en- ables us to measure only volatile constituents without the interference of involatile matters that we are neither interested in nor would like to introduce into the gas chromatographic system. It is also in good resemblance with human olfactory detec- tion. Isovaleric acid (C5HloO2) and caproic acid (C6H1202) were chosen as the target compounds to be quenched, since, as mentioned before, the former is the key odor component of foot odor, and the latter has been identified in axillary odor. The compounds under study are zinc oxide, aluminum chlorohydrate, spherical poly- ethylene powder, talc (Talc 15, Asada Seifun Co., Ltd.), benzalkonium chloride (C22H4oCIN, 50% aqueous solution, Toho Kagaku Co., Ltd.), HPZ, and HPA. Ten milligrams of each sample was weighed accurately in a glass vial especially designed for the headspace gas chromatograph, to which 2 ml of standard aqueous solution con- taining 0.3% of both isovaleric acid (Wako Pure Chemical Industries, Ltd., )98%) and caproic acid (Sigma Chemical Company, )99%) was accurately added. The hybrid powders Were also sampled to contain 10 mg as zinc oxide or aluminum chlorohydrate. The vial was tightly closed and placed inside an ultrasonic generator for 10 minutes for sample dispersion. It was then placed inside a 70øC oven for 60 minutes to equilibrate the vial headspace with acid vapor. The vial was introduced into a Hewlett Packard Headspace Sampler HP-19395A, which was attached to the injection port of a Hewlett Packard HP-5890 gas chromatograph equipped with a flame ionization detector and an HP-20 M (10 m X 0.53 mm i.d., film thickness 1.33 Ixm) column. The gas chro- matograph was programmed from 100 to 160øC at a rate of 10øC/min, with helium (10 psi) as carrier gas. The headspace of the vial was automatically pressurized, and a por- tion of it was forced into the carrier gas flow. The GC profile was recorded, and the peak areas were calculated in arbitrary units by an HP-5895A GC workstation, which also controlled the whole headspace GC system. For each sample, three consecutive GC runs were acquired, and the mean peak area was employed for the calculation described afterward. The standard acid solution was measured once in every five sample runs to ensure the stability of the headspace GC system. Each powder was evaluated by a value expressed as the odor suppression rate (OSR). OSR is calculated by the formula shown below. A-B OSR (%) - x 100 A A refers to the peak area of acid in standard acid solution B refers to the peak area of
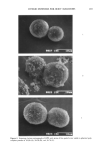
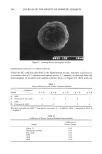
202 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS acid after addition of powder. An OSR value of 100 implies complete suppression of acid malodor. RESULTS AND DISCUSSION FORMATION OF HYBRID POWDERS Certain parameters were found to affect the physical appearance and thus the physical property of the resulting hybrid powder. These are the mixing ratio of core powder and outer layer powder, mixing time, and the size of mixing mediums. Examples of mixing ratios affecting the physical appearance of HPZ are demonstrated. Figure 4A is a microscopic profile of HPZ using 10 parts of fine-particle zinc oxide and 90 parts of spherical polyethylene powder. Some regions of the polyethylene powder surface are seen naked, suggesting that the amount of fine-particle zinc oxide was not enough. On the contrary, 50 parts of fine-particle zinc oxide and 50 parts of spherical polyethylene powder would overload the surface of polyethylene powder, as observed from Figure 4B. An ideal hybridization, where the surface of polyethylene powder is uniformly covered with zinc oxide, is shown in Figure 4C. This was accomplished when 30 parts of fine-particle zinc oxide and 70 parts of spherical polyethylene powder were hybridized. An ideal HPA was obtained also at a ratio of 30 parts of fine-particle aluminum chlorohydrate to 70 parts of spherical polyethylene powder. A micrograph of HPA is shown in Figure 5. Mixing time is also an important factor in producing hybrid powders. Sufficient mixing time is required, although unnecessarily long mixing time may lead to physical deteri- oration of the hybrid powder. Micrographs were taken every 10 minutes after com- mencing the mixing to monitor the progress of hybridization. For both HPZ and HPA, 30 minutes was found to be enough in our laboratory scale system. Regarding alumina ball size, 0.8 to 30 mm i.d. was found most suitable. TEXTURE EVALUATION OF HYBRID POWDERS The texture of hybrid powders evaluated by the direct evaluation method are summa- rized in Table I. HPZ and HPA were both found to possess preferred texture to their corresponding outer layer powders, i.e., fine-particle zinc oxide and fine-particle alu- minum chlorohydrate. The results show statistical significance by the Wilcoxon sign- rank test. The effect of hybridization was more remarkable for zinc oxide. The coefficients of kinetic friction measured instrumentally are summarized in Table II. The smaller the value, the smoother the texture. The coefficient of variation (cv %) for five consecutive measurements was below 5%. Nylon 12 powder (average particle size 6.6 I•m, Toray Industries, Inc.), our previous core powder, showed a value of 0.39, which was similar to that of spherical polyethylene powder. A drastic decrease in the values are observed when either fine-particle zinc oxide or fine-particle aluminum chlorohydrate is hybridized. Both hybrid powders gave values that were almost iden- tical with spherical polyethylene powder. The effect of hybridization was more remark- able with zinc oxide. These instrumentally measured figures were in excellent agree- ment with the results from the direct evaluation method.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)