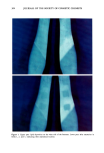
ANALYSIS OF SURFACTANTS 315 titrated with SDS. These same substances titrated with STPB showed between 4-10x higher potential jump (Figure 2) than when SDS was used as titrant (Figure 1). Figure 3 demonstrates a comparison between SDS and STPB used as titrants for coca- midopropylbetaine. Comparison of Curves 1 and 2 in Figure 3 indicates that STPB used as titrant gives a much more pronounced potential jump than SDS. This demonstrates that STPB is more electronegative than SDS. It is likely that the increased electronegativity in STPB is due to the localization of the negative charge on the boron atom, whereas in SDS the negative charge is distributed among the oxygens of the sulfate group. Thus, the ion-pair bond between cationic surfactant and STPB is stronger than between SDS, thereby demonstrating a higher potential jump. A disadvantage of STPB is its high price. An attempt to titrate the anionic moiety of cocamidopropylbetaine with benzethonium chloride was unsuccessful due to insufficient potential jump at the end point. Thus, this surfactant electrode can be used to titrate the cationic moiety of the coca- midopropyl betaine, whereas the carboxylic moiety is not a strong enough anion to provide a good potential jump. Figure 4 depicts titration of sodium polystyrene-sulfonate with benzethonium chloride. The above experimental data demonstrated that a surfactant electrode can easily be used as an analytical tool for quantification of polymeric cationic and anionic surfactants as well as betaine. SUMMARY The potentiometric procedure using a surfactant electrode has the following advantages: 1) It can be used for all types of ionic surfactant (polymer, hydrophilic, emulsion- forming), including betaines. 2) It has better precision and is less time-consuming than the ion-pair iso-extraction procedure. 3) There is no organic solvent waste. 4) No experience is needed to perform the assay. ACKNOWLEDGMENTS We are indebted to Paula and John Meehan of RLI Corporation for the funding to conduct this research. The support of Drs. David Cannell and Roger Mathews is gratefully acknowledged. REFERENCES (1) P. Mukerjee and A. K. Chosh, Isoextraction method and the study of the self-association of methylene blue in aqueous solutions. J. Amer. Chem. Soc., 92 (22), 6403-6407 (1970). (2) $. Motomizu, $. Fujiwara, A. Fujiwara, and K. Toel, Solvent extraction-spectrophotometric deter- mination of anionic surfactants with ethyl violet, Anal. Chem. 54, 392-397 (1982). (3) R. iV[. Good, J. C. Liao, iV[. J. Hook, and C. L. Panko, Colorimetric determination of a polymeric
316 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS quaternary ammonium antimicrobial preservative in an ophthalmic solution, J. Assoc. Off. Anal. Chem., 70 (6) (1987). (4) M. E. Del Rey and L. E. Vera-Avila, Adsorption of tetralkyl ammonium ions on reversed-phase HPLC columns, J. Liquid Chrom., 10 (13), 2911-2929 (1987). (5) N. Nakamura and Y. Morikawa, Separation of surfactant mixtures and their homologs by high performance liquid chromatography, J. Amer. Chem. Soc., 59 (1), 64-68 (1982). (6) B. M. Van Liederkerke, H. J. Nelis, W. E. Lamber, and A. P. DeLeenheer, High performance liquid chromatography of quaternary ammonium compounds on a polystyrene-divinylbenzene col- umn. Anal. Chem., 61, 728-732. (7) B. J. Birch and D. E. Clarke, Surfactant-selective electrodes, Anal. Chem. 67, 397-393 (1973). (8) K. M. Kale, E. L. Cussler, and D. F. Evans, Characterization ofmicellar solution using surfactant ion electrodes, J. Phys. Chem., 84, 593-598 (1980). (9) K. Vytras, Determination of some pharmaceuticals using simple potentiometric sensors of coated-wire type, Microchimica Acta (Vienna), III, 139-148 (1984).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)