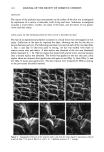
MODEL CREAM RHEOLOGY 79 160 120 25 øC 40• /'//"'/' •%,,.• ]primary .} spurs r ,,'"/,d" I I I I I 532 1064 1596 2128 2660 SHEAR STRESS (dynes/cm 2) Figure 1. Cream rheograms at 25 ø, 3 , 35 ø, and 40øC. to the left and the size of the hysteresis loop generally decreases as the temperature increases. The most predominant thermal effects are seen in the up curves of the rheograms, while the down curves vary relatively slightly. This would suggest that the rheology of the down curve is dominated more by mechanical stresses than by thermal stresses. Closer examination of the up curves reveals a characteristic inflection after the spur formation. The shear stress associated with this inflection shall be termed the inflection yield value, 'ri. As the temperature increases from 25-35øC, the inflection point shifts to a lower shear rate and becomes less defined. The associated 'r i value also decreases. Table I lists the inflection yield values and associated shear rates for each temperature. At 40øC the inflection totally disappears. As will be seen in the section on structure determination, the inflection point can be attributed to a secondary network Table I Inflection Yield Value Parameters Temperature Inflection yield value Inflection shear rate (øC) (dynes/cm 2) (sec- •) 25 1715 96 30 1569 82 35 1369 71 40 -- --
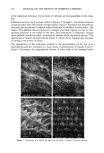
80 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS structure formed by the cetyl palmitate, while the other rheogram characteristics are due to the primary liquid crystalline gel network structure formed by the Lexemul AR © emulsifier system. STRUCTURE DETERMINATION Knowledge of the cream composition indicates that its structure is formed by a 2/1 ratio of Lexemul AR © to cetyl palmitate. In accordance with the gel network theory, the primary network structure of the cream is formed by the Lexemul AR ©, which is a commercial brand of self-emulsifying glyceryl monostearate having a cationic surfactant, stearamidoethyl diethylamine, as the emulsifier. As will become apparent from the thermal optical analysis data, the cetyl palmitate in the formulation precipitates with the Lexemul AR © during the cooling process of the cream manufacture and contributes to the network. A series of photomicrographs of the cream were obtained at 25 ø, 30 ø, 35 ø, and 40øC and showed no visual change in structure in response to the temperature change. Figure 2A shows the structure of the cream at 25øC. The numerous irregular-shaped particles were identified by their melting point to be cetyl palmitate. At 47øC the cetyl palmitate particles began to liquefy, and a slight volume expansion occurred. This portion of the melting transition was completed at 50øC and yielded oil droplets embedded in a matrix of Lexemul AR © as pictured in Figure 2B. The structure remained visually unchanged again until at 55øC the Lexemul AR © particles began to liquefy and coalesce. Rapid flow occurred at 59.5øC, indicating completion of the melting transition. Figure 3 shows the photomonitor recordings obtained during the heating program of the cream and the raw materials constituting its network structure. The photomonitor transition temperatures associated with these recordings are given in Table II. Exami- nation of the cream recording (Figure 3D) indicates that its melting transition shows an A B Figure 2. Photomicrographs of the cream at 500X magnification. A. 25øC sample prior to heating showing solid wax particles. B. 50øC sample showing molten cetyl palmitate droplets embedded in the Lexemul AR © matrix.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)