SOAP-INDUCED WINTER XEROSIS 207 form-methanol from corneocytes, proteins were extracted from corneocytes in 10 mM phosphate buffer, pH 8.0, containing 1% sodium dodecyl sulphate and 20 mM [•-mer- captoethanol at 60øC for 2 h. Aliquots were dried in microtiter plates at 85øC for 24 h and reconstituted in the BCA reagent (Pierce-Wariner, UK). Protein concentrations were determined by comparison with a protein calibration curve of bovine gamma globulin (Sigma Chemical Company, UK). EXTRACTION AND ANALYSIS OF DESMOGLEIN 1 (DSG 1) Corneocytes were extracted in 500 p•l of 50 mM Tris-HC1 buffer, pH 9, containing 8 M urea, 2% (w/v) sodium dodecyl sulphate, 5% (v/v) [3-mercaptoethanol, and 2 mM phenylmethylsulphonylfluoride (PMSF) at 90øC. After 15 min, an equal volume of 0.5 M iodoacetamide, in extract buffer, was added and incubated for a further 15 min at 90øC. The extract was diluted in 20 volumes of 50 mM Tris-HCL buffer, pH 7, containing 0.5 M sodium chloride, 1 mM manganese chloride, 1 mM magnesium chloride, 1 mM calcium chloride, 0.1% triton X100, and 2 mM PMSF (ConA buffer), and applied to 200-p•l columns of Concanavalin A-sepharose. The columns were washed with 20 ml ConA buffer, and the bound proteins were eluted with 500 p•l 0.5 M o•-methyl mannopyranoside in ConA buffer. 10 p•g of casein was added to the eluates as a carrier protein before precipitation of the eluates with four volumes of chloroform: methanol (1:4) followed by three volumes of water the precipitate was dried under nitrogen and reconstituted in sample buffer comprising 0.0625 M Tris-HCL, pH 6.8, containing 2% sodium dodecyl sulphate, 10% glycerol, and 20 mM dithiothreitol at 60øC, for 15 min. Samples were fractionated by sodium dodecyl sulphate polyacryl- amide gel electrophoresis (SDS PAGE) according to Laemmli (38) in 8% acrylamide gels (C = 3.3%) with a 4% stacking gel (C = 3.3%) the proteins were then blotted onto PDVF membrane by semi-dry electrophoretic transfer using a buffer of 39 mM glycine, 48 mM Tris-HCL, 0.0375% sodium dodecyl sulphate, and 10% methanol. The PDVF membranes were blocked overnight with 5% bovine serum albumin in 50 mM Tris-HCl, pH 7.5, containing 0.15 M sodium chloride, 2.5 mM potassium chloride, and 0.1% Tween 20 (TBS-tw). Desmoglein 1 was detected with rabbit anti- bovine Dsgl antiserum (1:100 R882.2 a gift from Dr. T. Egelrud) for 2 h, followed by incubation with a mouse anti-rabbit IgG monoclonal antibody conjugated to biotin (1:1,000 Zymed, UK) for 1 h and streptavidin-horseradish peroxidase conjugate (1: 1,000 Amersham, UK) for 30 min. Keratin was detected with mouse anti-keratin monoclonal antibody (Clone K8.13 Sigma) at 1: 50 to 1:200, as described above, except that a secondary antibody of sheep anti-mouse IgG-biotin (1:1,000 Amersham) was used. All incubations were performed in TBS-tw containing 0.1% bovine serum albu- min, and the washes between each step were with TBS-tw. The bands were visualized using the ECL chemiluminescence method (Amersham International, UK) and quanti- fied by scanning densitometry at 530 nm using a Schimadzu CS-9000 dual wavelength spot scanning densitometer. STATISTICAL ANALYSIS All results are represented as mean +-- standard deviation. Statistical comparisons were made using Student's t-test, and significance was set at the 5% level. Correlation
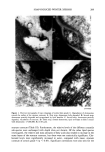
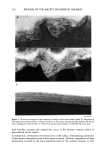
208 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS coefficients between each lipid component and the xerosis grades were determined using the Spearman correlation coefficient. RESULTS STRATUM CORNEUM LIPID AND DESMOSOME STRUCTURE IN NORMAL AND XEROTIC SKIN Sequential tape stripping of normal skin revealed morphological changes for stratum corneum lipids and desmosomes in the inner and outer layers of the stratum comeurn. In lower layers (third tape strip down) intact electron-dense desmosomal structures were seen (Figure 1D) in direct contact with the intercellular lipid lamellae. The desmosomes appeared to undergo degradation and a reduction in number in the upper layers of the stratum corneum. During their degradation, desmosomes showed digestion of their internal elements with vacuolation of their structures (Figure 1C) before detaching from the corneocyte envelopes. Desmosomal remnants often appear to be surrounded by intercellular lipids (Figure lB) before their total degradation (Figure 1A). The deeper tissue regions of winter xerotic stratum corneum resembled normal tissue. However, in contrast to normal skin, desmosomes persisted to the surface layer of the stratum corneum (Figure 2). The definition of a normal desmosome in these studies refers to desmosomes with electron-dense internal structures. During the tape-stripping proce- dure, desmosomes may be detached from adjacent cells, as in Figure 1D. In the lower layers of normal stratum corneum, lipids were present as multiple lamellae bilayer structures between the corneocytes (Figure 3C). However, toward the surface layers of the stratum corneum, the bilayer structures were no longer present, and appeared to have been degraded and replaced with a more amorphous-like structure (Figure 3A,B). In severe xerosis (grade 4 Figure 4), normal intercellular lipid structures were found in the lower layers of the stratum corneum (Figure 4C). However, in the peripheral layers of stratum corneum from subjects with severe xerosis, the normal lipid bilayer structure was replaced by large amounts of disorganized intercellular lipids (Figure 4A,B). STRATUM CORNEUM LIPID ANALYSIS An initial analysis of stratum corneum lipid composition from normal and xerotic skin was performed on pooled corneocytes from all of the tape strippings. Compared with normal skin (grade 1), statistically significant decreases in the mass levels of ceramide were seen in xerosis grades 3 and 4 (p 0.05), but not with grade 2 (Table I). Furthermore, the relative levels of the different ceramide species were unchanged (Table II). As changes in lipid structure were apparent in the outer layers of the stratum corneum in winter xerosis in the electron microscopy studies, we analyzed stratum comeurn lipids by depth, comparing the outer stratum corneum (first tape stripping) with the combi- nation of lipids in the remaining tape strippings (inner stratum corneum). No signif- icant difference was detected in the relative levels of ceramides in the outer and inner layers of stratum corneum in normal skin however, significant decreases in the relative levels of ceramide were seen in all grades of xerosis in the outer, compared with inner,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)