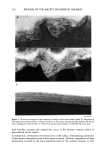
SOAP-INDUCED WINTER XEROSIS 205 The present study was designed to clarify the biochemical and morphological aberrations in stratum corneum lipids and desmosomes in skin xerosis. We investigated these components in particular because of the lack of consensus on the role of lipids in xerosis and because of the increased recognition of the role of desmosomes in stratum corneum cohesion and desquamation. The results demonstrate that stratum corneum ceramides are depleted in soap-induced winter xerosis however, there are no alterations in the relative ceramide sub-species. Additionally, we have shown that in soap-induced winter xerosis there was abnormal desmosomal degradation resulting in retention of these adhesive structures in the peripheral layers of the stratum corneum to produce skin scaling. MATERIALS AND METHODS SUBJECTS AND TREATMENTS Female Caucasians aged 30-40 years participated in this study. Subjects with genetic disorders or a history of atopy were excluded from the study (33), and all had normal skin at the start of the study. Normal controls were obtained from similar subjects not in the trial. To generate xerosis, all subjects refrained from the use of moisturizers and used only soap for cleansing three times per day for one week, as previously described (33), prior to grading and stratum corneum sampling. At the end of this period, the clinical severity of the xerosis of the dorsal surface of their hands was graded according to the following criteria: Grade 1: Normal skin. Grade 2: Mild xerosis characterized by small flakes of dry skin and whitening of dermatoglyphic triangles. Grade 3: Moderate xerosis, small dry flakes giving a light powdery appearance to the hand. Corners of dermatoglyphic triangles have started to uplift. Grade 4: Well-defined xerosis the entire length of a number of dermatoglyphic trian- gles have uplifted to generate large, dry skin flakes. Roughness is very evi- dent. STRATUM CORNEUM SAMPLINGS Skin biopsies were taken from the back of human hands using adhesive tape (Sellotape, DRG Products, UK). A 2 x 3-cm piece of tape was applied to the hands with gentle pressure by 20 strokes of a gloved finger and carefully removed. This procedure was repeated up to eight times on the same site, allowing analysis of the total stratum corneum sampled and stratum corneum of different depths the first tape was used to analyze the "outer stratum corneum" and the remainder pooled for the analysis of the "inner stratum corneum." Using electron microscopy techniques, it was found that approximately five layers of stratum corneum were removed during this process. Tape strippings were stored at -20øC until required. Corneocytes were detached from the tape in methanol with sonication, the tape was discarded, and the corneocytes together with methanol were dried under a stream of nitrogen.
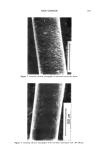
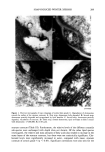
206 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ELECTRON MICROSCOPIC ANALYSIS Stratum corneum tape strippings were fixed for eight minutes in 0.1 M sodium caco- dylate buffer, pH 7.4, containing ruthenium tetroxide (0.2%), dehydrated through a series of alcohol solutions to propylene oxide and embedded in TAAB resin (TAAB Lab., Aldermaster, UK). Ultrathin sections were stained with lead citrate and viewed in a JEOL 100 CXII transmission electron microscope. STRATUM CORNEUM LIPID ANALYSIS All solvents were chromatography grade. Stratum corneum lipids were extracted from corneocytes using chloroform methanol (2:1) for two hours at RT, the solvent was aspirated from the corneocytes and dried under nitrogen, and the lipids were redissolved in chloroform. Stratum corneum lipid fractions were first chromatographically separated into their individual lipid classes and from tape stripping contaminants using solid- phase extraction columns. Briefly, the lipids were successively eluted from aminopropyl- bonded phase columns (Bond-Elut, Anachem Ltd, UK), using hexane to remove tape contaminants (neutral lipids were also lost in this fraction) hexane:ethylacetate (85:15) to elute cholesterol chloroform:isopropanol (2:1) to elute ceramides and finally, meth- anol:acetic acid (98:2) to elute fatty acids. The eluates were dried and redissolved in chloroform methanol (2:1). The isolated ceramide fractions and the combined choles- terol and fatty acid fractions were then quantitated separately by high-performance thin-layer chromatography (HPTLC). The HPTLC methodology was performed essen- tially according to Wertz et al. (34,35) and Ponec et al. (36), using silica gel 60 HPTLC plates (20 X i0-cm Merck, UK). Briefly, the ceramide and the cholesterol together with fatty acid fractions were chromatographically separated on separate plates by three sequential elutions of chloroform:methanol:acetic acid ( 190:9:1). After chromatographic separation, the lipids were visualized on the HPTLC plates by general degradative charring using an acidic copper sulphate solution (10% copper sulphate 8% phosphoric acid solution) and charring to 160øC for 20 minutes. After cooling, the lipid bands were quantified by reflectance densitometric scanning at 420 nm, using a Shimadzu CS-9000 flying spot densitometer (Shimadzu, Japan). Lipids were identified by their co- migration with cholesterol, palmitic acid, and N-stearoyl sphingosine standards (Sigma Chemical Company, Poole, UK). Ceramide subclasses were also identified by the rf values from the literature (21,34-36) together with their chromatography characteristics similar to those of the standard ceramide. Ceramide one was further identified by its change in chromatographic mobility after alkali hydrolysis (34) and by co-migration with chemically synthesized ceramide one, the identity of which had been verified by mass spectroscopy and proton-NMR. The mass of each stratum corneum lipid was determined from the appropriate series of standards chromatographed on each plate. Triglycerides and other neutral lipids, together with cholesterol sulphate levels, could not be determined, as tape-stripping contaminants co-migrated with these lipids during chromatography. To allow inter-person comparisons, the mass of each lipid fraction was normalized to the amount of stratum corneum removed by tape stripping. The amount of stratum cor- neum was estimated by quantification of the detergent-soluble protein of the corneocytes extracted under reducing conditions (37). Briefly, following aspiration of the chloro-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)