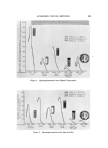
796 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS It is generally regarded that air bubbles in cosmetic preparations are incorporated into the products during manufacturing operations involv- ing entrainment of the surrounding air. However, very little work has been done in this area to determine the exact mechanism of aeration. Entrainment of the surrounding air into a liquid due to vortex forma- tion in a mixing operation is, of course, well-known and is probably the most common source of aeration in cosmetic preparations. In some cases, vortex formation may be desirable in order to facilitate wetting of a powdered material it is generally to be avoided since it promotes aer- ation and encourages evaporation of volatile components. When a pro- peller or turbine mixer is used, it is generally possible to eliminate the vortex by employing baffles or placing the mixer off-center (3). The surrounding air can also be entrained without a vortex if the fluid is in turbulent flow. The eddies and irregularities of the fluid sur- face produced by rapid mixing, pumping, or pouring operations can often be responsible for aeration. However, Lin and Donnelly have pointed out the possibility of air entrainment by smooth jets in laminar flow (4). Such possibility can exist in filling of viscous lotions using a circular noz- zleo Another possible source of air bubble entrainment occurs during a pumping operation when there is a leakage in the suction side of the line. However, this possibility, as well as others mentioned thus far, involves entrainment of the surrounding air and the air incorporated into the product originates externally. Although it is possible to produce gas bubbles in cosmetic products through internal generation of gases, there appear to be few publications concerning such a mechanism in practical situations. It is theoretically possible to generate a gas in a product either as a result of a chemical reaction liberating the gas or a decrease in dissolved gas solubility. Since most cosmetic materials are quite inert, it would be unusual for a chemical reaction to take place during the manufacturing operation or shelf-life resulting in liberation of a gas. However, internal generation of gas bubbles due to a decrease in solubility characteristics probably occurs more frequently in cosmetic processing than is sus- pected. Since the solubility of a gas usually decreases with temperature, heat- ing of a liquid saturated with a gas generally results in liberation of the dissolved gas. However, it is also possible to generate gas by mixing two liquids together under an isothermal condition. To explain, Fig. 1 il- lustrates a solubility curve of a gas, X, in a binary mixture of AB solu-
BUBBLES IN GELS 797 25 F, 2.01 o _ o x _ 0 25 50 I00 (100%0) % A IN AO SOLUTION (0%B) Figure 1. Solubility curve of gas X in AB solution tion consisting of liquids A and B. The solubility curve of this gas, rep- resented by the solid line, is curved upward since the system is not an ideal solution. According to Fig. 1, if one takes 100 g of liquid A satu- rated with gas X (solubility = 2 g/100 g of A) and mixes it with 100 g of liquid B saturated with gas X (solubility = 1 g/100 g of B), he would have a total of 2 -+- 1 or 3 g of gas X in the system. However, this mix- ture, which is approximately a 50% solution of A (49.7%, to be exact), can dissolve only 1.2 g of the gas per 100 g of the liquid or 2.4 g per 200 g of the mixture. Therefore, approximately 0.6 g of the gas must escape from the mixture in order to establish an equilibrium under an isothermal condition. The above illustration implies that if the solubility of a gas in a mix- ture of two liquids is less than the value expected from the linear inter- polation of the solubilities in pure components, the gas can be liberated by mixing two saturated solutions together. While making clear Carbopol ©* gels containing water and alcohol, it was found that no matter how much care was exercised to prevent the externally entrained air, depending on the method of preparation, the finished gel could contain a considerable amount of air. This mecha- nism was considered a possible cause for the bubble formation and ex- periments were conducted to explore this theory using hydroalcoholic Carbopol gels. * Carbopol resins, B. F. Goodrich Chemical Co., Cleveland, Ohio.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)