798 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS EXPERIMENTAL In most experiments, 0.5% Carbopol 940 was used as the thickener to form hydroalcoholic gels. An equal amount of either Ethomeen C/2• ©* or triethanolamine was used as the neutralizer for Carbopol 940. De- natured, anhydrous ethyl alcohol (S.D.A. 40) or pure ethanol and deionized water were used in various proportions. In the experimental investigation, it was first assumed that the Car- bopol resin used had nothing to do with bubble formation except that its thickening action would trap the bubbles and prevent them from escap- ing into the atmosphere. If this were true, and if the mechanism involving a solubility de- crease as illustrated by Fig. 1 were indeed responsible for air bubble for- mation, one would observe generation of air bubbles when alcohol satu- rated with air was mixed with water saturated with air. Furthermore, the amount of air generated by such a mixture should be roughly equal to the amount trapped in the gel made by presaturating the water and alcohol with air. To test this hypothesis, two types of experiments were conducted. The first involved collection and measurement of the amount of air liberated by mixing various proportions of ethanol with water without a gelling agent under an isothermal condition. The second involved preparation of a series of Carbopol gels with varying water/ethanol proportions and determination of the bubble content after the systems had reached an equilibrium. A lcohol-Water Mixing Experiments The simple mixing of ethanol with water produced tiny gas bubbles which were visually observed. After the generated gas was collected and analyzed by a gas chromatographic method, it was confirmed that the liberated gas was air. However, since mixing of ethanol and water involved generation of heat, it was necessary to remove this heat to maintain the system under an isothermal condition. Figure 2 illustrates the setup used in the alcohol-water mixing ex- periments. The mixing chamber consisted of a 508-ml flask equipped with a precision thermometer, cooling coil, and a magnetic stirrer. The cooling coil was connected to a refrigerated constant-temperature bath through a pump, and the flow rate of the cooling water controlled by * Ethomeen C/25 (polyoxyethylene coco amine), Armour Industrial Chemical Co., Chi- cago, Ill.
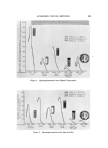
BUBBLES IN GELS 799 THERMOMETER BURET MAGNETIC Figure 2. Experimental setup regulating the valve opening. A capillary tube, fitted on the top of the flask, led the generated gas into an inverted buret where the volume of the gas was measured. The liquid used in the buret and the bubbling bath was identical to the alcohol-water solution in the mixing flask. The temperature of the system was kept at 23 øC during the experiments. To start the experiment, water and ethanol, both presaturated with air and kept at 23 øC, were weighed separately. Ethanol was first placed in the mixing flask and then water was added. The cooling water was then turned on to overcome the temperature rise as a result of the heat of mixing. When the temperature of the mixture reached 23 øC, the stop- per was placed tightly on the mixing flask and the magnetic stirrer was turned on at a low speed. The undissolved air liberated would start to bubble at this time and flow into the buret. The volume of the air col- lected was determined by taking the buret reading when all bubbles were liberated and correcting for the hydraulic pressure. The result was ex- pressed in terms of a, defined as ml of air liberated at 1 arm, 23 øC, per 100 g of the water-alcohol mixture. The values of a were determined at varying alcohol contents. Because the mixing of alcohol and water would produce a volume contraction, this reaction might affect the measurement of the trapped air. Fortunately, the volume contraction occurred much more rapidly than the liberation of the undissolved air and it was found that as long as the water was added to alcohol, and not vice versa, enough mixing would take place to give a nearly full contraction before the stopper was placed. For this reason, no volume correction was needed to take ac- count of volume contraction.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)