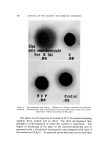
800 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Gel Experiments In the second series of experiments, hydroalcoholic Carpobol gels having various alcohol contents were prepared. Before forming a gel, Carbopol 940 resin was dispersed in the alcohol and the neutralizer was dissolved in the water. These solutions were then saturated with air by bubbling in air at 23 øC. A gel was then prepared by combining the two phases under carefully controlled mixing to avoid entrainment of any external air. The gel was kept in an enclosed jar and placed in a con- stant temperature bath at 23 øC for about 24 hours or until an equi- librium was reached. In the systems studied, it generally took about 2 minutes after gel formation before bubbles started to appear. Both the number and the size of bubbles increased slowly with time until a maximum was reached. This generally required several hours. When the density of the gel (in- eluding all air bubbles) reached a constant value, the system was con- sidered to have reached an equilibrium. The amount of air bubbles in the system was then calculated from the density of the aerated gel and the density of a corresponding bubble-free gel. The results were also expressed in terms of a, the volume occupied by the bubbles in ml at equilibrium per 100 g of gel at 23 øC. As in the alcohol-water mixing ex- periments, a was determined as a function of the ethanol content in the gel. Before taking samples for density measurements, the aerated gel was mixed completely to assure the uniformity of the bubbles in the gel samples. Two to four samples were taken from various locations of each gel system and the bubble distribution was considered uniform when the densities of the samples agreed well. RESULTS AND DISCUSSION The results of the alcohol-water mixing experiments are given in Fig. 3 by a solid line. Considering the difficulty in maintaining the system at a constant temperature and preventing bubble escape before the stop- per was placed, the data were fairly consistent and reproducible. Ac- cording to the curve, the amount of air released increases with the alcohol content to a maximum value and then starts to decrease as the mixture approaches pure alcohol. The results of the bubble formation in hydroalcoholic gels are also presented in the same graph by a broken line. Although these two curves do not coincide exactly, it is interesting to note that the shapes of the curves are very similar and both curves show maximum values at
BUBBLES IN GELS 801 Figure S. o 0 IOO ALCOHOL-WATER MIXTURE / / O O O O 20 40 60 80 % ALCOHOL Results of alcohol-water mixing and Carbopol gel experiments ethanol content around 55%. It is possible, therefore, that the same mechanism may be responsible for air bubble release in both experiments. The volume of air in the Carbopol gels was calculated by taking the difference between the reciprocal of the density of aerated gel and that of bubble-free gel. Since, in the systems studied, the densities of the aerated gels were not greatly different from the densities of the cor- responding bubble-free gels, the results were very sensitive to small errors in measurements. For this reason, the data from the gel experiments were not as consistent as the data from the alcohol-water mixing experi- ments. Moreover, some loss of bubbles could not be completely pre- vented in the gel experiments during the mixing operation and equili- brating period. Although the extent of bubble loss is believed to be small, it could have contributed to the lower values of a in the gel experiments. Assuming that an identical bubble-formation mechanism is in opera- tion, another explanation for a lower a value in gel experiments may be formulated by considering surface tension. It is known that bubbles in a fluid are subjected to a pressure additional to the static pressure as the result of surface tension. This pressure is directly proportional to sur- face tension but inversely proportional to the diameter of the bubble (5). Therefore, the smaller the bubble, the larger will be the pressure inside the bubble. Many bubbles in the Carbopol gels were microscopic in dimensions and they were, therefore, subjected to this additional pres- sure which would make the total volume of bubbles smaller than the true volume at atmospheric pressure. This effect has no influence on the measurement in alcohol-water mixing experiments since the volume
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)