802 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS was measured after all the undissolved air was collected in the buret. It is possible, therefore, that this effect was responsible for the smaller a value obtained in the gel experiments. It was reasoned that if the dissolved-air liberation mechanism due to mixing of the alcohol and water as illustrated in Fig. 1 were indeed re- sponsible for the bubble formation in the Carbopol gels, one should be able to prepare a nearly bubble-free gel by changing the manufacturing procedure. Since Carbopol resins do not gel until neutralized, any bub- ble formed prior to neutralization could escape from the mixture. Therefore, instead of neutralizing the Carbopol gel immediately after the water and alcohol are mixed, one could prepare gels by delaying neutrali- zation after the undissolved air in the alcohol-water mixture had a chance to escape. Theoretically, a Carbopol gel so prepared should contain no bubbles (although it may still contain some dissolved air) l•rovided that no external air is incorporated during the preparation. Similarly, .one should be able to prepare a bubble-free gel if all dissolved air were first removed by heat, vacuum, ultrasonic generator, or other means. To test the above theory, Carbopol gels containing 50% ethanol, 0.5% Carbopol 940, 0.5% triethanolamine, and 49• water were made using the following procedures: Procedure I (Control): Carbopol 940 was dispersed in ethanol, and triethanolamine was dissolved in water. The two solutions were mixed with a propeller mixer to form a gel instantly. This was the procedure used in the gel experiment. Procedure II (Delayed neutralization A): Carbot•ol resin was dispersed in a preblend of alcohol-ethanol. The dispersion was allowed to stand for several hours to free air bubbles before neutralization. Procedure III (Delayed neutralization B): The Carbopol resin was dispersed in alcohol, and water was added. The solution was mixed thoroughly and the mixture was allowed to stand for several hours to free air bubbles before neutralization. Procedure IV (Ultrasonic method): The Carbopol resin was dis- persed in alcohol, water was added, and then this mixture was passed through a laboratory ultrasonic homogenizer.* The homogenized mix- ture stood for 10 minutes to free cavitation bubbles before neutraliza- tion. As expected, the gel from Procedure I contained a considerable amount of air bubbles. The gels from Procedures II and III contained * Minisonic IV Ultrasonic Emulsifier, Sonic Engineering Corp., Norwalk, Conn.
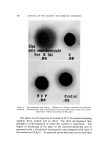
BUBBLES IN GELS 803 -• •'•-•. 7 ..... " ß • • .... • ...................... :• •" ":" '7., '•' :: • ..•'.•,. • ....... ........... . . - . ... ...,•.• ..... :. • -. . •:•-...• •......• . - ..• '• .•.z.:.. ß .: :'..'•" i.•: 2 .... • ..... .::.... ...:•:: ' .. Figure 4. Carbopol gels prepared by four different procedures (1 = control, 2 = delayed neutralization A, 3 = delayed neutralization B, 4 = ultrasonic method) only a very small amount of air bubbles and the gel from Procedure IV contained virtually no air bubbles. The photograph in Fig. 4 shows the samples taken from this series of experiments. The number on the bottle corresponds to the procedure used. From the results obtained, the theory of dissolved air appears to be a plausible explanation for the observed bubbles in Carbopol gels. The formation of such bubbles can be prevented in Carbopol gels by a delayed neutralization technique either with or without deaerating equipment such as an ultrasonic machine. However, the cavitation produced by an ultrasonic unit is very effective in removing the dissolved air and such a machine can be very useful in shortening the time required to free the undissolved air. In order to study the time required to free the undissolved air, another series of experiments was conducted with the same Carbopol system using Procedure III. The Carbopol resin was first dispersed in the alcohol using a propeller mixer. After all the bubbles in the dis- persion had escaped, water saturated with air was uniformly blended into the mixture in a large flask. The total weight of the mixture was 2 kg. Immediately, a 2-in. propeller mixer was placed in the solution to agitate the mixture at about 300 rpm. Samples of 100 g each were taken at various intervals after mixing was started and the neutralizer was added to these samples to produce gels. The mixture was covered during the mixing to prevent evaporation. These gel samples were placed in a constant temperature bath at 24 øC and their densities were determined after an equilibrium was reached.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)