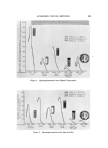
8O4 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS o .905 •BLE FREE / GEL. 50% ETHANOL 0.5ø/ø CARBOPOL 940 0.5% T.E.A. 49ø/ø WATER 60 120 MIXING TIME BEFORE NEUTRALIZATION (MIN ) Figure 5. Effect of delayed neutralization The results of these experiments presented in Fig. 5 indicate an increase in gel density (i.e., decrease in the amount of bubbles) with the mixing time before neutralization until a maximum value is reached. The graph shows that after 2 hours of mixing, practically all the air had escaped from the system as the gel sample gave a maximum density of 0.9176. Visually, this gel showed very few air bubbles. Since the releasing of the undissolved air is dependent on the degree of agitation, the temperature of the system, etc., the result presented in Fig. 5 cannot be directly used to predict the extent of deaeration in a large processing vessel. However, the data clearly demonstrate the effectiveness of the delayed neutralization technique. It is interesting to note that by coupling this technique with the use of an ultrasonic machine, the mixing time was reduced from 2 hours to 10 minutes in making a bubble- free gel. CONCLUSIONS Experimental data presented indicate that in the absence of ex- ternally entrained air, hydroalcoholic Carbopol gels can form bubbles depending on the method of gel preparation. There was strong evidence suggesting that the observed bubble formation resulted from internal liberation of the :undissolved air following a reduction in air solubility in the system. The Carbopol polymer itself did not appear to affect bubble formation except that, when neutralized, the gel trapped the liberated air to produce bubbles '
BUBBLES IN GELS 805 The answer to bubble formation in Carbopol gels due to such a mechanism is quite simple. Since the bubbles result from the undis- solved air produced in the system when alcohol and water are combined, a logical solution is to wait until all the undissolved air is liberated before neutralizing the gel. Using such a technique, one can prepare a bubble-free hydroalcoholic Carbopol gel provided that no air is entrained from the surroundings. It was demonstrated that the amount of air bubbles in the finished gels could be controlled by varying the mixing time prior to neutraliza- tion. Apparently, mixing encourages liberation of the undissolved air in the system. In preparing such gels in production scale, the use of an ultrasonic homogenizer may be advantageous. By a cavitation process, such a machine can remove the dissolved air from an ethanol- water mixture within a short time and help to yield bubble-free gels. Furthermore, such a unit may also help to disperse Carbopol resins more completely and prevent lump formation. ACKNOWLEDGMENT The author gratefully acknowledges the experimental assistance of Tony Lew, Max Factor & Co. (Received February 17, 1969) REFERENCES (1) Martin, A. N., and Banker, G. S., Rlzeology, in Bean, It. S., Beckett, A. H., and Carless, J. E., Advances in Pharmaceutical Sciences, Vol. 1, Academic Press, New York, 1964, p. 58. (2) Lin, T. J., Rheology Fundamentals and Application in Cosmetic Industry, in deNavarre, M. G., Chemistry and Manufacture of Cosmetics, Vol. 1, Van Nostrand, Princeton, N.J., 1962, pp. 329-36. (3) Oldshue, J. ¾., Fluid mixing of cosmetic formulations, J. Soc. Cosmetic Chemists, 10, 332-40 (1959). (4) Lin, T. J., and Donnelly, H. G., Gas bubble entrainment by plunging laminar liquid jets, A.J. Ch.E. Y., 12,563-71 (1966). (5) Adamson, A. W., Physical Chemistry of Surfaces, 2nd ed., Interscience Publishers, New York, N.¾., 1967, pp. 2--4.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)