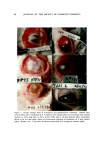
96 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS cultures were incubated with excised normal corneas. Results with endotoxin paralleled those with P. aeruginosa qualitatively, although there was a quantitative reduction in lytic activity. The fact that col- lagenolytic activity is not seen when excised normal corneas are incubated with P. aeruõinosa or with endotoxin suggests that an enzyme activator is needed. The activator may be produced in vivo during a pseudomonas infection or in response to intracorneal injection of endotoxin. This needs to be confirmed. DISCUSSION These experiments show that under conditions involving defined numbers of pseudomonas organisms and certain defects in the corneal epithelium, rabbit eyes develop pseudomonas keratitis. The effects ap- pear to be more serious in rabbit eyes than in monkey eyes, and the se- quence of destructive events progresses more rapidly in rabbits. It is not possible from these limited studies to say conclusively which species is more representative of man. Nevertheless, the effects are sufficiently similar in both species to warrant concern about their application to man, particularly with regard to current eye-decorating practices among women. A report from Wilson et al. (12) states that the outer eyes of women who use eye cosmetics often harbor pseudomonas organisms which are transferred to their eye liners, mascaras, and eye shadows, where growth may continue. Later, these potentially infectious materials are reapplied to the eyes. This situation could have unpleasant consequences, es- pecially for contact lens users, whose eyes may more readily suffer slight penetration of the corneal epithelium. In this connection, it is note- worthy that a combination of pseudomonas contamination and corneal injury from a commercial shampoo (which was subsequently removed from the market) was capable of initiating pseudomonas keratitis in rabbits. For some time, the Food and Drug Administration has been concerned about the inadequacy of preservative systems in cosmetics (1 g). Mercury, which is not condoned in other cosmetics (14), is currently tolerated in eye-area cosmetics (15), pending the development of better preservative systems for eye preparations. This decision is based on the fact that mercury is markedly effective against pseudomonas organisms in these eye cosmetics (16). Furthermore, systemic mercury poisoning and skin sensitization from these amounts of mercury are unlikely.
PSEUDOMONAS KERATITIS 97 The finding that P. aeruginosa does not readily invade vascular tis- sues of rabbits such as sclera and skin appears to parallel the human ex- perience. Pseudomonas infections have been reported to occur in human skin (17), however. (Received August 23, 1971) REFERENCES (1) Gordon, D. M., and McLean, J. M., Colistin in pseudomonas infection, Arner. J. Ophthalrnol., 50, 33 (1960). (2) Hessburg, P., Truant, J., and Penn, W. P., Pseudomonas Corneal Ulcers in Animals and Man, in Sylvester, J. C., Antimicrobial Agents and Chemotherapy, American Society for Microbiology, Ann Arbor, Mich., 1964, pp. 752-8. (3) Duke-Elder, S., System of ophthalmology, Vol. 8, Part 2, Diseases of the Outer Eye, C. V. Mosby, St. Louis, 1965, pp. 782-4. (4) Olson, S. R., The application of microbiology to cosmetic testing, J. Soc. Cosmet. Chem., IS, 191 (1967). (5) Dunnigan, A. P., Microbiologic control of cosmetics, Drug Cosmet. Ind., 102(6), 43 (1968). (6) Ribi, E., et al., Preparation and host-reaction properties of endotoxin with low content of nitrogen and lipid, ]. Exptl. Med., 114, 647 (1961). (7) Boivin, A., and Mesrobeaner, L., Recherches sur les antigenes somatiques et sur les endotoxins des bacterius. I. Considerations generales et expose des techniques utilises, Rev. Immunol., 1, 553 (1935). (8) Webster, M. E., et al., Studies on the "0" antigen of Salmonella typhos. I. Purification of the antigen, ]. Irrtrnunol., 74, 455 (1955). (9) Fisher, E., and Allen, J. H., Corneal ulcers produced by cell-free extracts of Pseudo- domonas aeruginosa, Amer. ]. Ophthalmol., 46, No. 1, Pt. 2, 21 (1958). (10) Morihara, K., Pseudornonas aeruginosa proteinase, Biochirn. Biophys. Acta, 78, 113-24 0963). (11) Mandl, I., MacLennan, J. D., and Howes, E. L., Isolation and characterization of pro- teinase from Cl. histolyticurn, J. Clin. Invest., 32, 1323 (1953). (12) Wilson, L. A., et al., Microbial contamination in ocular cosmetics, Arner. J. Ophthalrnol., 71, 1298 (1971). (13) Bruch, C. W., Microbiological quality to topical products, Drug Cosmet. Ind., 109(1), 26 (July 1971). (14) FDA Trade Correspondence No. 412, dated February 11, 1944, in Kleinreid, V. A., and Dunn, C. W., Federal Food, Drug and Cosmetic Act: Judicial and Administrative Record 1938-49, Commerce Clearing House, Inc., Washington, D.C., p. 740. (15) Private communication with John Gowdy, M.D., Division of Cosmetics, Bureau of Foods, Food and Drug Administration, regarding a proposed Federal Register Statement for cosmetics containing mercury, August 1971. (16) Eckhardt, W., ?henyl mercuric compounds as preservatives for cosmetics, Arner. Per[urn. Cosmet., 85(3), 83 (1970). ,q7) Hall, J. H., et at., Pseudomonas aeruginosa in dermatology, Arch. Dermatol., 97, 312 (196s).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)