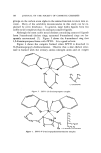
100 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS example, in chloroform, ,to 0.34% and in dimethylformamide, to 0.81%. The solubility in dimethylsulfoxide is reported to be 5.13% (2). Ac- cordingly, it has been difficult to formulate suitable cosmetic composi- tions containing solubilized ZPTO. Although the sodium salt of pyrithione is highly soluble in water, and like ZPTO has been found to have fungicidal and bactericidal properties, it, unlike ZPTO, has been found toxic and unacceptable for use in cosmetics or dermatological compositions for topical application to the skin (3). HYPOTHESES It was postulated that the solubility of ZPTO would be increased in solvents containing amino functional groups. The reasoning for this is based upon the ability of certain heavy metals such as cadmium, zinc, copper, and nickel to form ammine complexes. Zinc has a coordination number of 4 which means that it can attach itself spatially to 4 ligand linkages besides maintaining its two primary valence linkages. In the molecular structure of ZPTO (Fig. 1), two of the four available spatial positions are filled by the oxygen in the oxide functional groups of the molecule. A chelate is formed. By selecting the proper amine solvent it was hoped that the chelate structure would be modified to allow for the accommodation of a molecular complex containing amino groups that would utilize the remaining two Werner bonds. Figure 1. Zinc pyrithione (zinc pyridine-2-thiol-l-oxide) (ZPTO) One of the first materials to be tested was reagent grade 28% am- monium hydroxide. It was found that ZPTO was soluble in it to the order of 1 or 270. Although this in itself was not considered significant, it proved somewhat interesting when a 1% solution of ZPTO in am- monium hydroxide was used to neutralize aqueous Carbopol 941.* A clear gel resulted that had a pH of 8 and contained 100 ppm or 0.01% ZPTO. When the pH of the gel system was lowered, it was found that * B. IV. Goodrich & Co., Cleveland, Ohio.
ZINC PYRITHIONE PREPARATIONS 101 the growth of small, fine crystals had formed throughout the media. Thus, what in effect we were able to do was to prepare a near-saturated, saturated, or supersaturated solution of ZPTO relatively quickly and without the presence of an equilibrating solid. The mechanism by which ZPTO produces a therapeutic effect on seborrheic dermatitis is not understood. If one postulates that the mechanism of the action of ZPTO is through epidermal penetration, then one must conclude that it is not the ZPTO particles but rather the molecules themselves that penetrate the epidermis. One can then reason that the use of a saturated solution of ZPTO can give correspond- ing effects as a suspension of the solid in contact with its supernatant liquor. If a 1% suspension of ZPTO contains only 0.01% (100 ppm) available material for epidermal penetration, then the remaining 0.99% dispersed solid can be considered unavailable for penetration into the skin. Hence, theoretically, the clear Carbopol gel that was prepared containing 0.01% solubilized ZPTO can be just as efficient a preparation in relieving seborrheic dermatitis as an opaque preparation containing 99-fold more ZPTO as suspended material. This statement is conjec- ,ture and no attempt has been made to determine its validity either experimentally or clinically at this time. EXPERIMENTAL The procedure for determining solubility was rapid, but only ap- proximate, since the ultimate end of the testing was not the elucidation of solubility data, but the formulation of hair products. Thus, 1 g of ZPTO was dissolved into 9 g of the amine in question with gentle heating, if necessary. The ease with which the ZPTO was dissolved was noted along with the quantity of residual insolubles at room tempera- ture. An estimation of the solubility of ZPTO in the amine was then made. Besides ammonium hydroxide, the following common labora- tory amines were tested for their solvent effect on ZPTO: triisopropanolamine triethanolamine N-methyldiethanolamine N N-dime thylaminoethanol N-ethyldiethanolamine N,N-diethylaminoethanol These amines have several characteristics in common. All are tertiary amines they have difunctionality in the form of alkanol groups that
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)