102 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS lower their basicity through an inductance effect they are all very poor solvents for ZPTO. Other amines which proved ,to be poor solvents were trilaurylamine, ethoxylated cocoamine-15 moles ETO, and morpholine. It was found that the following compounds containing primary amino functional groups made excellent solvents: n-dodecylamine 1,3-diaminopropane N-(3-aminopropyl) 1,3-propanediamine N- (3-aminopropyl)N-methyl- 1,3-propanediamine bis(2-aminoethyl)sulfide All of these amines were capable of dissolving over 10% ZPTO. There were also a number of oxyamino compounds that showed very good solvent properties. These were: ethanolamine diglycolamine 3-methoxy-n-propyl amine 3-(2-ethoxyethoxy)-n-propyl amine 3-[ 2-(2-e thoxye thoxy)e thoxy] -n-pro pylamine Diglycolamine,* or 2-hydroxy-2'-aminodiethylether, was singled out for its unique solvent properties: it can dissolve 42% ZPTO. Of course, the fact that this amine itself was water soluble was also con- sidered favorable. In relation to the ethoxylated propylamines, it was observed that increasing the ethoxylation function lowers their solvent capacities. Thus, while 3-(2-ethoxyethoxy)-n-propylamine was able to dissolve over 10% of ZPTO, the solubility capacity of 3-[2-(2-ethoxy- ethoxy) ethoxy]-n-propylamine was approximately 8%. A number of primary amines that were expected to be good solvents but failed to be were: N,N-dimethyl- 1,3-propanediam ine N-(3-aminopropyl)cyclohexylamine bis(3-aminopropyl)piperazine 4-(3-aminopropyl)morpholine ethylenediamine cyclohexylamine * Jefferson Chemical Co., Houston, Tex.
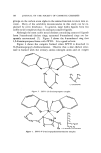
ZINC PYRITHIONE PREPARATIONS 103 DISCUSSION AND APPLICATION The solubility of ZPTO in organic amines may be looked upon as the interaction of a Lewis acid and a Lewis base in which the ZPTO, acting as a Lewis acid, accepts a pair of electrons from an amino group thus forming a covalent bond. Zinc is a class a metal, that is, it is an electropositive metal that forms its most stable complexes with ligands in which the donor atom is nitrogen, oxygen, or fluorine (4). The sta- bili•ty of the resulting metal complex is a function of the nature of the ligand. With class a metals, the greater the base strength of the ligand the greater is the tendency to form stable complexes. However, this was found not to be the case in all instances. According to dissocia- tion constant tables, cyclohexylamine, with a pK• of 10.64, should be equal to if not a better solvent for ZPTO than dodecylamine (pK• 10.62) (5). It was shown that cyclohexylamine was a considerably poorer sol- vent than dodecylamine. Ethanolamine, with a relatively low pK• of 9.50, was capable of dissolving ZPTO to over 30%. The high solvency of ethanolamine can be explained through chelation. The formation of a chelate structure usually lends itself to stabilizing the complex, and the more extensive the chelation, the more stable the system. Fig- ure 2 shows a possible three-dimensional chelate structure of ethanol- amine. Observe the formation of a 5-membered chelate ring and note that the spatial arrangement of the coordination bonds allows zinc to be positioned in the center of a tetrahedral structure (6). However, 2-amino-2-methylpropanol, which can be thought of as a dimethyl deriv- ative of ethanolamine, NHsC (CHa)sCHsOH, is an extremely poor sol- vent. This is not surprising if the steric hindrance of the two methyl ETHANOLAMINE Figure 2. ZPTO-ethanolamine complex
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)