108 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS AZIRID•NE H POLYETHYLENIMINE (PEI) oeigure 7. Acid-catalyzed polymerization ooe aziridine to form polyethyleneimine What made this material unusual as compared to other amines which had been tested was the fact that water or alcohol was actually required in order to dissolve ZPTO. In effect, we were now able to make clear preparations of ZPTO that contained large quantities of water. Ini- tially, it was found that 1 part ZPTO can be conveniently dissolved in water using 2 parts polyethylenimine of molecular weight 600 (PEI). The resulting solution, based upon a 20% ZPTO concentration, had a pH of 10.6 and could be diluted to infinity with water without causing the precipitation of ZPTO. It was later determined that 1 part ZPTO can be so]ubilized with as little as 1.35 parts PEI. When the pH of an aqueous solution containing 270 ZPTO and 470 PEI was lowered by the slow addition of 3.7•o hydrochloric acid, a precipitate occurred be- low a pH of 8.8. The precipitate was identified as ZPTO by infrared spectroscopy and was shown to have the same spectra as the crystals growing in the diglycolamine-Carbopol hair dressing. In a study of the structure of the complex it was shown that the ultraviolet absorption spectrum of a dilute aqueous solution of ZPTO shifts to one similar to sodium pyrithione when aqueous PEI is added (10). Since sodium pyrithione is an ionic salt and has no coordinate covalent metal linkages, the indication is ,that the chelate bonds within the ZPTO molecule are broken. From this inference, the solubilization of ZPTO by aqueous PEI can be accounted for by either of two ex-
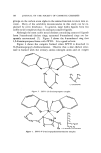
ZINC PYRITHIONE PREPARATIONS 109 Figure 8. Possible structures of solubilizc{ ZPTO in aqueous PEI planarions. The first (Fig. 8,A) is that a complex is formed with PEI that is analogous to the tetraammonia zinc complexes. For this to happen the two coordination linkages that form the internal chelate structure of ZPTO would have to open and allow the total of four co- ordination linkages of zinc to form with PEI. The second explanation (Fig. 8,B) is that of a decomposition of ZPTO into a pyrithione salt of PEI and zinc which would be complexed in alkaline PEI media. It• this is the case, then a solution of ZPTO in aqueous PEI may exhibit toxic pharmacological properties similar to sodium pyrithione and therefore render its use in cosmetic compositions unacceptable. To determine the nature of the PEI-solubilized ZPTO, a series of chromatographic techniques was employed in which gel filtration studies were prominent. Gel filtration, a form of column chromatography, separates substances by molecular weight. The higher the molecular weight, the more rapidly the substance is eluted from the column. ZPTO has a molecular weight of 318 compared to approximately 600 for the PEI which we used. If the two are not complexed they should separate on the column into two peaks. It was found that PEI, zinc, and pyrithione migrate as one peak. This phenomenon indicates that the zinc and the pyrithione exist in chemical combination in the PEI sys- tem. This conclusion was also sustained from data obtained from thin- layer chromatography studies.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)