122 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table I Accuracy of Methylene Chloride Determination Methylene Chloride Known (%) Found (%) Standard I 10.12 Standard II 10.32 10.11-4-0.17 ( 7 detns) 10. 324-0.32 (10 detns) Table II Analysis of Commercial Hair Spray Samples Showing Reproducibility of Method Precision Methylene Chloride (%) (1 Sigma) (%) Sample A 7.224-0.15 (8 detns) 4-2.08 Sample B 8.104-0.15 (7 detns) 4-1.79 ferred to the sampling bottle as previously described. One-tenth milli- liter of n-propanol per gram of sample was added. The results of 8 determinations on sample A and 7 determinations on sample B are shown in Table II. The precision of the method is shown to be +---2% relative. Because it is not always known how much methylene chloride is contained in an unknown sample, it was desired to determine the rela- tive response factor over a wide range of methylene chloride concentra- tions while holding the n-propanol concentration constant. The con- centration of methylene chloride was varied from 3 to 20% by weight of hair spray. The samples were analyzed in the normal manner. The ratios of the areas of methylene chloride and n-propanol were plotted against the ratios of their weights. The linear curve shown in Fig. 4 indicates that the response of the detector is linear over the range which might be expected to be encountered in hair sprays. Several observations were made during the course of the investiga- tion. After many injections into the hot vaporization zone, the polymer carbonized in the needle making operation of the plunger difficult. The syringe was dismantled and the plunger wiped clean with alcohol-soaked tissue. Pulling a fine crimped wire through alcohol and the needle several times effectively removed the carbon deposit inside the needle. A leak developed at the seat of the needle into its Teflon seal. This was remedied by cutting a I/s-in. long piece of Teflon tape from a 1/2-in. wide piece and stretching it to about 2 in. in length and wrapping it around the stem of the needle against the metal stop before inserting it into the seal and securing.
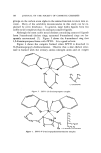
GAS CHROMATOGRAPHY OF METHYLENE CHLORIDE 123 A R E 2.0 A 1.0 Me CL z/n, - PROPANOL I.O 2D 5.0 WEIGHT Figure 4. Response of detecto,' to methylene chloride relative to n-propanol over range ooe ale, proximately 3 to 20% in hair spray It was observed that samples in the serum bottles could be inverted in a small beaker and held several hours at room temperature without change in composition. Maintaining these samples overnight, either at room temperature or at refrigerator temperature, was not successful. •towever, a series of samples held overnight in a freezer gave identical results when reanalyzed the following day. After 30 or 40 samples were run through the column, a slight distor- tion of the n-propanol peak and deteriorating resolution were observed. This was atributed to a build-up of plasticizer and other higher boiling ingredients in hair spray on the column. Conditioning the column at 200øC for 1/2 hour at the start of a day's run was found to eliminate this problem. There has been no indication as yet of any failure of the column after almost 500 analyses. SUMMARY A new gas chromatographic method for determining methylene chloride in hair sprays in less than 10 min has been developed. The reproducibility of commercial samples containing 7-8% methylene chlo- ride has been shown to be better than --2% relative. Expensive sample equipment or instrument modifications are not required and training of personnel is minimal. (Received October 1, 1971)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)