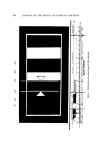
PHYSICAL ASPECTS OF FOAM 309 FRACTIONATION Overflowing foam involves foam fractionation. Dissolved (or col- loidal) surface-active components of the liquid are adsorbed at the bubble surfaces and carried off by the foam. A surface-inactive com- ponent often can be adsorbed through union with an appropriate sur- face-active collector. Figure 4 illustrates simple foam fractionation, batch and continu- ous, with gas injection. If the submergence of the bubbler in the liquid pool is not too shallow, and the foam is stable, the solute surface excess, r•v, on the bubbles will be approximately in equilibrium with the solute concentration in the pool, C,•. The foam fractionator then operates approximately as a single theoretical (perfect) stage of separation. Overflow Focm Foam Liquid Feed Liquid Overflow Bottoms.. Gas 1 • Gas • (a) (b) Figure 4. Foam fractionation operating in the simple mode: (a) Batch, (b) Flow A solute material balance on the foam gives 3GF•v co = c., + ½2) where CQ is the solute concentration in the overflowing foam after it is collapsed, and 3/r is the ratio of surface to volume for a bubble. By combining eq 12 with a material balance over the entire fractionator under steady continuous operation, one obtains 3GF•v Cw = Cv rF (13)
310 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS where F is the volumetric flow rate of feed liquid, and CF is the solute concentration in the feed. With nonuniform bubble sizes, r in eqs 12 and 13 is replaced with ra.2. Careful feeding into the foam at some level above the pool, so that the feed drains down countercurrently through the ascending foam, pro- duces a stripping action which further lowers Cw. Collapsing the over- flowing foam mechanically, and returning all or part of it as reflux to the upper region of the ascending foam, produces an enriching action which further concentrates the overflow that is, it elevates Ca (25). Surface loss within the rising foam, which can result from bubble wall rupture or interbubble gas diffusion, furnishes internal reflux. Strip- ping and enriching can also be carried out simultaneously. These higher modes of operation can be analyzed by means of transfer units and limiting equations (26). For further information regarding foam fractionation, including list- ings of many substances which have been separated and discussions of some of the chemistry involved, the reader is referred to several reviews on the subject (27-31). Foam fractionation is one of the adsorptive bubble separation pro- cesses (32, 33) [abbreviated as adsubble processes (32)]. For a thorough discussion of this entire class of processes, the reader is referred to a recent comprehensive book (34). (Received November 2, 1971) REFERENCES (1) Bikerman, J. J., Foams and emulsions, Ind. Eng. Chem., 57 (1), 56-62 (1965). (2) Hoffer, M. S., and Rubin, E., Flow regimes of stable foams, Ind. Eng. Chem., Fundam., 8, 483-90 (1969). (3) Shih, F. S., and Lemlich, R., Continuous foam drainage and overflow, Ibid., 10, 254-9 (1971). (4) deVries, A. J., Foam Stability, Rubber-Stichting, Delft, 1957. (5) Mysels, K. J., Shinoda, K., and Frankel, S., Soap Films, Pergamon, New York, 1959. (6) Leonard, R. A., and Lemlich, R., A study of interstitial liquid flow in foam. Part II. Experimental verification and observations, AIChE J., 11, 25-9 (1965). (7) Miles, E. D., Shedlovsky, L., and Ross, J., Foam drainage, J. Phys. Chem., 49, 95-107 (1945). (8) Clark, N. O., The electrical conductivity of foam, Trans. Faraday Soc., 44, 13-5 (1948). (9) Fanlo, S., and Lemlich, R., Predicting the performance of foam fractionation columns, AIChE--Inst. Chem. Eng. (London) Syrup. Ser. 9, 75-8, 85-6 (1965). (10) Jashnani, I. L., Coalescence and HTU in Foam Fractionation Columns, Ph.D. disserta- tion under R. Lemlich, Univ. Cincinnati, 1971. (11) Leonard, R. A., and Lemlich, R., A study of interstitial liquid flow in foam. Part I. Theoretical model and application to foam fractionation, AIChE J., 11, 18-25 (1965).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)