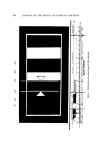
PHYSICAL ASPECTS OF FOAM 303 I-X 0.20 0.16 0.12 0.08 0.04 / 0 0.02 0.04 0.06 0.08 0.10 Kf/K• Figure 2. A plot of (1 -- X), the volumetric fraction of liquid in foam, versus Kt/K •, the ratio of foam conductivity to liquid conductivity, based on the average of results from sev- eral investigations (3, 8-10) The ease with which light can be transmitted through foam increases with the average bubble size (12). However, as a means for actually esti- mating the average bubble size, this method can be questioned (lg). Other factors also affect the transmissivity of light. The initial average volume per bubble can be found from the gas rate and the bubble frequency by measuring the latter stroboscopically (6). However, photography is a more general method in that it records individual bubble sizes as well. With suitable magnification, film and PB dimensions also can be measured. The foam to be photographed can be frozen and then sliced (14), or, more conveniently, photogTaphed as is at a free surface or through a transparent bounding surface such as that of a glass container. The dis- tribution of bubble sizes in a surface layer is reportedly almost the same as the distribution in the bulk foam (14). However, questions of indi- vidual bubble distortion and optical uncertainties at the bounding sur- face remain, although some recent experiments by the author's group in- volving the building of a foam one bubble at a time seem to indicate that these problems may not be serious, at least for foams made of large bub- bles of uniform size (10). With foams of widely varying bubble size, a more important matter is the statistical difference between the true bubble size distribution in the foam and the apparent distribution at any surface. This difference stems from the greater probability of a large bubble being intercepted by the surface, as compared to a small bubble being intercepted. The prob- ability is proportional to the bubble radius.
304 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Accordingly, this bias can be corrected by weighting the distribution at the surface inversely with the bubble radius (4). The final results can be expressed succinctly as rj,• = r'j-•,•-• (3) where, for the true distribution, •nr • rj,• •-• - •nr• (4) and r'j-l,k-1 is defined correspondingly for the surface. Symbol n repre- sents the number of bubbles of radius r. Thus, for example, the true ordinary arithmetic mean radius rl,0, which is defined as ,•nr/,•n for the true distribution, equals r'0,-1 which is ,•n/,•(n/r) from the surface distribution. It can also be shown that the true frequency distribution function of radii can be found from the dis- tribution function at the surface by multiplying the latter by r'o,_•/r. STABILITY Foam stability is ascribed to several factors. Before a bubble wall rup- tures, it first stretches and thins locally. This action increases the local surface area, thus momentarily decreasing the concentration of surfactant adsorbed at the surface. This decrease o.ccurs partly because the supply of surfactant within the film is limited, and partly because the surfactant requires some finite time to diffuse from the interior of the film to the surface. The resulting local increase in surface tension tends to draw the stretched (thinned) film together again, thus opposing rupture. The "healing" of the film which is based on the limited supply of sur- factant is sometimes termed the Gibbs effect, and that which is based on the diffusional lag is sometimes called the Marangoni effect. The relative importance of these two effects is still subject to dispute. With ionic surfactants the electrostatic repulsion between the parallel surfaces of the film opposes thinning and thus contributes to stability. High surface viscosity and high liquid viscosity also contribute to stability by damping the effect of local disturbances and by slowing drainage. Possible sources of disturbance include pressure fluctuation, thermal fluctuation, spontaneous vapor nucleation, ionizing radiation, external vibration, and internal stresses brought about by local changes in the bubble packing that result from the changes in bubble size caused by gas diffusion between bubbles. This last phenomenon will be discussed presently.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)