POLYAMIDE RESINS FOR HAIR 323 bonded to the hair to give improved body. The size and number o[ curlers to be used depend on the amount of curl desired. For some purposes, only limited curl is desired and is used for its effect on ap- parent body of the hair, not to give a curl per se. Synthesis of the aminopolyamide and its reaction with epichloro- hydrin to give the reactive azetidinium chloride derivative are shown in Fig. 1. HOOC(CH,04COOH + H•NCH2CH2NHCH2CH•NH2 H [NH•(CH2) 4 •N m cg2cm2$-- cg2cg 2 Ix o o Epichlorohydrin © c1 © [NHC(CH,)4CNHCH2CH•/N. CH2CH2]x O O aC•c•CH2 I OH •igure 1. Aminopolyamide synthcsis and reaction with epichlorohydrin As shown in Fig. 2, ammonium thioglycolate reacts with the disulfide groups o[ hair to generate sulfhydryl [unctionality. Reaction o[ the resin with sul[hydryl groups is shown in Fig. 3. The sketch shows only one o[ the thioether linkages. Since both resin and hair protein are poly[unctional, the resin may be bonded at more than one point. Conditions for applying the resin to hair and completing the reac- tions were studied by use o[ 2-g hair tresses, which were also useJul [or observing the effects on ease o[ styling, style retention, improved body, S S S S S S S S SHCH2C02NH4 H I H s s s Disulfide crosslinked Protein with disulfide hair protein linkages partially reduced to sulfhy dryls Fi,•ure 2. Reaction of ammonium thio•lycolate to generate reactive sulfhydryl groups
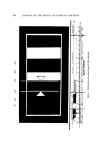
324 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS s s s Hair prot•ein with sulfhy dryl groups C1 © N HC0(CH2)4CONHCH2 CH 0 H Reactive resin • c1 o I HCOH •sin molecule I CH• I s I s s I s s Hair protein S I (•sin molecule) Resin bonded by thioether linkage Figure 3. Bonding of resin to hair by fortnation of thioether linkages and elasticity. The same effects were then studied by treating hair on mannequins, which afforded a better means for observing ease of styling and style retention. The following example illustrates how the resin was applied to 2-g hair tresses and also defines conditions used later for in vivo applications. The tress was shampooed and rinsed, blotted with a towel to a moist condition, and then wetted thoroughly with a 9% solution of ammonium thioglycolate adjusted to pH 9.0 with ammonium hydroxide. The wet tress was wound on a medium-size, cold-wave rod and remoistened with this glycolate solution. After 5 min, the tress (still on the rod) was rinsed thoroughly, then wetted with a 1.5% solution of the polyamide conditioner. After 2 min, the tress was wetted with an oxidizing solu- tion (1) containing 7% sodium bromate and 4% sodium dihydrogen phosphate with pH adjusted to 6.5 to 7.0. The rods were removed after
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)