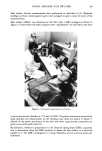
264 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS EXPERIMENTAL MATERIALS [1-•4C] lauryl alcohol was obtained from New England Nuclear Corp. (Boston, Mass.) and purified by silica gel chromatography. The specific activity was 12.1 mCi/mmole. Polyoxyethylene [1-•4C] lauryl ether (•C-LAEO) was prepared as follows: [1-•C] lauric acid (New England Nuclear Corp.) was reduced to [1-•C] lauryl alcohol with purity not less than 99%, to which ethylene oxide (EO) was added to synthesize an addition product of 7 mole EO on average. The product was subjected to silica gel chromatography and preparative thin layer chromatography to obtain the following LAEO: •C-LAEO-1, 1 mole EO addition •4C-LAEO-2-76, average 2.6 mole addition (a mixture of LAEO-2 and -3) and •4C-LAEO-•--.•, average 6.4 mole addition consisting of various LAEOs from -2 to -14. The specific activity was 4.35 mCi/mmole. •C-LAEO-10 (average 10 mole EO addition), synthesized by Daiichi Pure Chemicals Co. (Tokyo, Japan), was purified by silica gel column chromatography. It consisted of EO additions of not less than LAEO-4. The specific activity was 3.72 mCi/mmole. Unlabelled LAEOs used were as follows: lauryl alcohol was a reagent grade material of Tokyo Kasei Co. LAEO-1, -2, and -3 were commercial products and were purified by silica gel chromatography. LAEO-6.4 and LAEO-I• were synthesized at our laboratory and purified by silica gel chromatography under the same conditions as •C-LAEO. ANIMALS Hairless mice were obtained from the National Institute of Genetics (Japan) in 1968 and have been kept and bred at our animal laboratory. Of these, 10 weeks old female mice (18-20 gr.) were used. PERCUTANEOUS ABSORPTION A hairless mouse was immobilized by its paws and tail on a stainless steel restrainer. A silicone resin enclosure (1.0 cmx 2.9 cm) was glued to the dorsal skin with o•-cyanoacrylate. Twenty five #1 of •4C-LAEO ethanol solution (55.2 mmole/1) was applied to the skin (1.0 #Ci, 1.38 #mole), and the mouse was immediately put into a container to measure expiratory excretion, as shown in Figure 1. The air was supplied to the container at the rate of 300 ml/min. through 20% NaOH aqueous solution and saturated saline solution. The expired air was filtered through a 50% monoethanol- amine-methanol solution. At the end of the experiment the animal was sacrificed, and the skin of the applied area was excised. MEASUREMENT OF THE AMOUNT IN THE BODY The body of a hairless mouse less the excised skin from the product application area was put in a blender, and 40 ml of iN NaOH aqueous solution were added. The body was homogenized in the blender for 10 minutes. An aliquot (1-1.5 gr.) of the homogenate was dried and cornbusted for radioactivity determination. This method provided recovery of greater than 98% of the •4C activity in the body of the hairless mouse.
PERCUTANEOUS ABSORPTION OF SURFACTANTS 265 Air pump T 20% NaOH solution solution T T , T Saturated Flow Closed plastic 50% monoethanolamine NaCl meter container methanol solution (300m•/min) (3•) Figure 1. Schematic diagram showing 14CO2 collection in expired air. MEASUREMENT OF EXPIRATORY EXCRETION Carbon dioxide in the expiratory excretion was absorbed in 50% monoethanolamine- methanol solution (15 ml, 2 traps). Radioactivity was measured in the whole solution. This system gave a recovery of 14CO2 not less than 99%. MEASUREMENT OF EXCRETION IN FECES AND URINE This experimental system could not provide for separation of feces from urine thus, the feces and urine samples were jointly, respectively dried, combusted, and counted. MEASUREMENT OF THE AMOUNT REMAINING IN THE SKIN APPLICATION SITE •4C activity in the excised skin was extracted by methanol solution and then by toluene. •4C activity remaining in the extracted skin was measured by combustion of the skin after drying. ANALYSIS OF •4C All samples were counted with a Beckman LS-233 liquid scintillation spectrometer. Monoethanolamine-CO2 and extracted solution were dissolved in a liquid scintillator (5 gr. ppo, 0.1 gr. popop in 1 I toluene) for radioactivity measurement. Samples of body homogenate, feces and urine, and skin were combusted on a Sample Oxidizer (Packard Tri-carb, Model 306) to prepare for liquid scintillation counting. RESULTS AND DISCUSSION PERCUTANEOUS ABSORPTION OF LAEO Table I summarizes the intravital amount, fecal and urinary excretion, expiratory excretion, and percutaneous absorption four hours after the topical application of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)