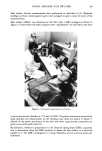
268 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 100 ,::, • 80 • o • F-- 60 I..,t.I • el.,. • ,,'I, qo ILl Z 2O (1) (2) LAURYL ALCOHOL 0 1 2 3 q TIME (HOURS) LAEO-1 I I I I 0 1 2 3 4 TIME (HOURS) 10 LAEO-2.6 LAEO-10 0 1 2 3 q 0 q 8 12 2q TIME (HOURS) TIME (HOURS) Figure 3. Time-course of per cent excreted in expiration. Applied dose: 1.38/amole/25/al ethanol (1.0 /aCi). Dorsal skin of hairless mouse: 2.9 cm 2. Each point is the mean of 3 animals. •4CO2 of expiratory excretion Per cent excreted in expiration = •4C_LAEO absorbed percutaneously depicted in Figure 3. The percent excreted in expiration was defined as the activity of •4CO2 excreted in expiration divided by the activity of •4C-LAEO absorbed percuta- neously. For lauryl alcohol (Figure 3.1) and LAEO-1 (Figure 3.2), the percent excreted in expiration increased greatly during the course of time when the percutaneous absorption was in a steady state. This indicates that the LAEO absorbed percutaneously is not rapidly excreted in expiration. However, the percent excreted in expired air was nearly constant for LAEO-2.6 (Figure 3.3) and LAEO-T0 (Figure 3.4), thus the percutaneous absorption was in a steady state. In these cases, the LAEO absorbed percutaneously was rapidly metabolized to CO2 and excreted in expiration. Therefore, the expiratory excretion was proportional to the percutaneous absorption in LAEO-2•. and LAEO-10. The rates of cumulative expiratory excretion are depicted in Figure 4. The results indicate linearity. The expiratory excretion rate was obtained from the slope of the lines and then used to attain the percutaneous absorption rates which are constant. The rates
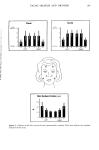
PERCUTANEOUS ABSORPTION OF SURFACTANTS 269 A o 12 xlO 4 lO _ ,• LAEO-2,6 . . LAEO-10• _• ••0•• • m 0 1 2 3 q 5 6 TIME (HOURS) Figure 4. Cumulative expiratory excretion of LAEO. Applied dose: 1.38/zmole/25/zl ethanol (2,220,000 dpm). Dorsal skin of hairless mouse: 2.9 cm 2. thus obtained are shown in Table IV. These values are in good agreement with the values obtained from time course changes in the percutaneous absorption shown in Table III. This expiratory excretion is greatly affected by changes in the chain length of EO units of LAEO. In Table V, the percent excreted in expired air four hours after topical application is summarized: 60% of the lauryl alcohol absorbed percutaneously was excreted in expired air and only 2.6% in feces and urine. We assume that lauryl alcohol is absorbed into the body, metabolized, and excreted in expired air with '4CO 2 as the main route. When one unit of EO is added to lauryl alcohol, the percent excreted in expiration is reduced from 60% to 28%, but increases in feces and urine from 2.6% to Table IV Rates of Percutaneous Absorption of LAEOs Rate of Percutaneous Absorption Compound (/zmole/cm 2. hrs) -- LAEO-2.6 10.2 _+ 0.6 x10 -2 -- LAEO-10 0.79 + 0.08 x 10 -2 The absorption rates were determined by measurement of the rate of excretion in expiration (from Figure 2).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)