EFFECTS OF IRRITANTS ON SKIN 199 In previous studies we analyzed the effects of treatment of mouse epidermis with TPA on the profile of newly synthesized proteins using two-dimensional polyacrylamide gel electrophoresis (13). Over 200 individual proteins were resolved in acidic gels and at least 10 of these showed major (five-fold or more) increases or decreases in response to TPA. Several of these proteins appeared to be keratins which are important markers of epidermal cell differentiation. The present study was designed to investigate whether the tumor promoters anthralin and benzoyl peroxide produced similar changes in the production of mouse epidermal proteins. Our results demonstrate that these compounds produce distinct effects on epidermal protein synthesis, suggesting that there may be several alternate mechanisms of tumor promotion in the mouse skin. EXPERIMENTAL Animals used were female, CD-t mice 7-t0 weeks of age, supplied by Charles River Breeding Laboratories. Anthralin was provided by Elder Pharmaceuticals. Benzoyl per- oxide was purchased from Aldrich. TPA was obtained from Consolidated Midland Corp. Each chemical was dissolved in acetone (Fischer) and freshly prepared for each experi- ment. 35S-methionine (tOO0 Ci/mmole) was purchased from New England Nuclear. Ampholines for isoelectric focusing gels were from LKB, Sweden. Tissue culture me- dium and newborn calf serum were obtained from Grand Island Biological Company. LABELING OF EXCISED EPIDERMIS Twenty-four hours before application of the compounds the dorsal skins of the mice were shaved. TPA, anthralin, and benzoyl peroxide were applied in 0.2 ml of acetone directly to the shaved skin. Control mice received 0.2 ml of acetone alone. Twenty- four hours after treatment, the mice were sacrificed by cervical dislocation, and a chemical depilatory (Neet, Whitehall Laboratories) was applied for 3 minutes. The depilatory was removed from the skin by washing the animal under cold water. The skin was then excised and the subcutaneous adipose tissue was removed with a scalpel. The sample was cut into 4 sections (approx. 2 cm 2 each) and floated dermal side down in 3 ml of incubation medium in 5 cm plastic tissue culture dishes. The incubation medium consisted of Dulbecco's Modified Eagle's Medium, without methionine, sup- plemented with 5% newborn calf serum and tOO •Ci/ml 35S-methionine. The culture dishes were then incubated at 37øC in a humidified CO2 incubator. After 4 hours, pure epidermis was harvested by the heat treatment method of Raineri et al. (14) and immediately placed in 0.4 ml modified O'Farrell lysis buffer (15) consisting of 9.5 M urea, 2% w/v Nonidet P 40, and 5% 2-mercaptoethanol. The resulting lysate was sonicated for t minute on a Sonic Dismembrator (Artek Corp., Model 150). Samples were then stored at -70øC until use. For protein analysis, samples were centrifuged for 5 minutes in a Fischer micro-cen- trifuge (model 235) at approximately 10,000 x g. Only clear supernatant fractions .were used. Protein was determined by the method of Bradford (16), using bovine serum albumin as the standard. Radioactivity was measured on a Tracor Mark III scintillation counter.
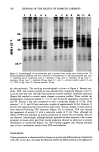
200 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS POLYACRYLAMIDE GEL ELECTROPHORESIS One-dimensional discontinuous SDS-polyacrylamide slab gels were run according to the method of Laemmli (17). The separating gel contained 10% polyacrylamide. Du- plicate gels were run for autoradiography and protein silver staining (18). Samples for silver staining contained 10-50 p,g of protein, and for autoradiography, approximately 100,000 cpm. Two-dimensional polyacrylamide gel electrophoresis with isoelectric focusing was carried out as described by O'Farrell (15) with some modifications. Am- pholines, pH 5-8 and pH 3.5-10, were used in the equilibrium isoelectric focusing dimension, and discontinuous 10% polyacrylamide gels were used in the second di- mension. 1.6% ampholines (pH 5-8) and 0.4% ampholines (pH 3.5-10) were added to the epidermal samples prior to first dimension electrophoresis. Pre-stained molecular weight standards (Bethesda Research Labs.) used were cytochrome C (molecular weight 12.3 kd), [3-1actoglobulin (18.4 kd), tx-chymotrypsinogen (25.7 kd), ovalbumin (43 kd), bovine serum albumin (68 kd), phosphorylase B (92.5 kd), and myosin H-chain (200 kd). Gels were fluorographed using EnHance (New England Nuclear) to visualize radioactive peptides. RESULTS Application of TPA (10 •g) or anthralin (0.1-80 •g) to mouse skin resulted in marked irritation characterized by hyperemia and increased epidermal thickening. These effects were observed within 24 hours after treatment. Anthralin-treated skin was also discol- ored with a characteristic yellow-brown appearance. In contrast, we found that 24-hour treatment with benzoyl peroxide (1-40 mg) did not induce any of these changes in the skin, even at the highest dose tested. In order to determine if the increased epi- dermal thickening was associated with altered protein production, we examined the effects of these compounds on epidermal protein synthesis. Skin fragments were pulse- labeled with 35S-methionine and extracts prepared as described in the Experimental section. Aliquots of these extracts were acid precipitated and counted for radioactivity. We found that anthralin caused a significant, dose-related decrease in protein synthesis in the range of 0.1 to 80 •g/mouse (Figure 2, Table I). At 80 }xg, the maximum dose tested, protein synthesis was inhibited by 78%. This dose of anthralin corresponds to the optimal concentration required for tumor-promotion in mouse skin (4). In contrast, with tumor-promoting doses of benzoyl peroxide (40 mg, see Table I) there was a 15% stimulation of protein synthesis. TPA (10 •g/mouse) produced a 37% decrease in protein synthesis. In an attempt to determine if specific proteins were being altered, we analyzed the protein profiles of the control and treated epidermis using one-dimensional polyacryl- amide gel electrophoresis. Parallel gels were run and stained with silver for analysis of total protein content or subjected to autoradiography for determination of newly syn- thesized proteins (see Experimental section). Silver-stained gels revealed that treatment of mouse skin with promoting agents produced no significant effects on protein dis- tribution (not shown). Autoradiographs of these gels, however, revealed several major differences between anthralin and benzoyl peroxide (Figure 3). Anthralin (40-80 •g) treatment induced the disappearance of a protein with a molecular weight of approxi- mately 60 kd, and the appearance of several proteins in the molecular weight range of 45-50 kd. These findings are similar to those observed with TPA (Figure 3 and
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)