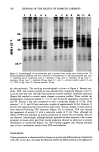
EFFECTS OF IRRITANTS ON SKIN 203 IEF = IEF • 789 ,! :, t10 -68 .. - '46.- '-"' ,..2 ":. -43 I -25.7 Figure 4. Two-dimensional gel profiles of proteins synthesized by control and anthralin-treated mouse epidermis. Samples from •SS-methionineolabeled extracts (containing 1 x 105 cpm of labeled protein) were analyzed on two-dimensional gels, with isoelectric focusing (pH gradient 5-7) in the first dimension and SDS-polyacrylamide gel electrophoresis in the second dimension. Gels were exposed to x-ray film for 14 days. Each spot was assigned an arbitrary number, as indicated. (a) Control mouse skin. (b) Anthralin (80 Ixg) treated skin. -25.7 Figure 5. Two-dimensional gel profiles of proteins synthesized by benzoyl peroxide and TPA-treated mouse skin. (a) Benzoyl peroxide (40 mg)-treated skin. (b) TPA (10 Ixg)-treated skin. of high abundance keratin proteins. These proteins have molecular weights in the range of 45-70 kd. In previous work using two-dimensional gel electrophoresis we have shown that TPA can alter the production of keratins. These are proteins 7, 8, 9, 10, 43, 46, and 51, indicated on the gels in Figures 4 and 5. The coordinate changes in
204 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS all of these proteins are unique to TPA and are not observed with other irritant, hyperplasiogenic agents including ethylphenylpropiolate, acetic acid, and turpentine oil (13). In the present paper we sought to expand these studies and determine whether the alterations in protein production were common to other promoting agents. The tumor promoters evaluated were anthralin and benzoyl peroxide. We found that anthralin, but not benzoyl peroxide, was able to mimic nearly all of the effects of TPA on mouse skin proteins. These included changes in the production of the same keratins, except for protein 51, and a similar reduction in protein 2, the identity of which is not known. When compared to TPA, anthralin was a more potent inhibitor of epidermal protein synthesis, This could be accounted for, at least in part, by the inhibition of keratin biosynthesis. Using one- and two-dimensional gels, we also found that overall production of protein was inhibited non-selectively. At the present time, the basis for the lowered rate of protein synthesis after anthralin and TPA treatment of mouse skin is not apparent. Inhibition of' protein synthesis in epi- dermal cells in culture by TPA has been reported (20). It is possible that this step may be required for tumor promotion induced by these agents. In the case of anthralin, this phenomenon may account for its anti-psoriatic activity (21). The failure of benzoyl peroxide to induce changes in epidermal protein synthesis is surprising in view of its known promoting activity. It is known that benzoyl peroxide is unstable and breaks down rapidly when applied to skin (22). However, Slaga et a! (3), have shown that a single promoting dose of benzoyl peroxide (40 mg) when applied topically to the backs of mice induced epidermal hyperplasia and morphologic changes similar to those caused by TPA. There is evidence that peroxides and free-radicals may be intermediates in tumor promotion induced by TPA (23) and that benzoyl peroxide may work directly in this fashion. Peroxides have also been shown to damage DNA directly and to be mutagenic (24). It is possible that benzoyl peroxide-induced tumor promotion represents a distinct mechanism in chemical carcinogenesis. Further studies are necessary to clarify this mechanism. REFERENCES (1) N. L. Lowe and J. Breeding, Anthralin, Arch. Dermato/., 117, 698-700 (1981). (2) S.C. Harvey, "Antiseptics and Disinfectants Fungicides Ectoparasiticides," in The Pharmacological Basis of Therapeutics, 6th ed., A. G. Gihnan, L. S. Goodman, and A. Gilman, Eds. (Macmillan Publ. Co., New York, 1980), p 974. (3) T.J. Slaga, A. J. P. Klein-Szanto, L. L. Triplett, L. P. Yotti, and J. E. Trosko, Skin tumor- promoting activity of benzoyl peroxide, a widely used free radical-generating compound, Science, 213, 1023-1025 (1981). (4) A. Segal, C. Katz, and B. L. VanDuuren, Structure and tumor-promoting activity ofanthralin (1,8- dihydroxy-9-anthrone) and related compounds, J. Med. Chem., 14, 1152-1154 (1974). (5) I. B. Weinstein, L. S. Lee, P. B. Fiscl-ter, A. Mufson, and H. Yamasaki, Action of phorbol esters in cell culture: Mimicry of transformation, altered differentiation, and the effects on cell membranes, J. Supratool. Struct., 12, 195-208 (1979). (6) I. Berenblum, "Sequential Aspects of Chemical Carcinogenesis: Skin," in Cancer.' A Comprehensive Treatise, F. F. Becker, Ed. (Plenum Press, New York, 1975), Vol. 1, pp 323-344. (7) A. N. Raick, Ultrastructural, histological and biochemical alterations produced by 12-0-tetradeca- noyl-phorbol-13-acetate on mouse epidermis and their relevance to skin tumor promotion, Cancer Res., 33, 269-286 (1973). (8) T. S. Argyris, The regulation of epidermal hyperplastic growth, CRC Critical Rev. Toxicol. 9, 151- 2OO (1981).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)