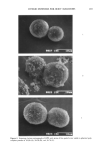
AXILLARY MALODOR 177 solution was obtained, and after cooling the product was precipitated with 300 g eth- anol. The white crystals were washed three times with absolute ethanol and dried in vacuo. Zinc as determined by atomic absorption was 27% (theoretical, 30%), and gly- cine by Kjeldahl was 68% (theoretical 70%). Solubility of zinc glycinate (Zn-GLY) in water at 20øC and pH 7.2 was found from zinc concentration of a saturated solution to be 5 g/100 mi. METHODS PILOT STUDY A double-blind screening study was designed to detect the enzymes beta-G and AS in axillary secretions. Swabs were taken from the axillae of twenty men, classified by professional odor evaluators (Hilltop Laboratories, Cincinnati, OH) as 10 "high-odor formers" and 10 "low-odor formers." Trypticase soy agar plates were prepared con- taining either 4-MUS (substrate for aryl sulfatase) or 4-MUG (substrate for beta-glu- curonidase) at a concentration of 25 ppm. Elutions of the swabs were plated on both substrates, incubated for 24 hrs, and the fluorescence of the plates was estimated visually. SEMIQUANTITATIVE ASSAYS OF BETA-GLUCURONIDASE AND ARYL SULFATASE The enzyme assays measure conversion at 37øC of the non-fluorescent substrates 4-MUG (for beta-G) and 4-MUS (for AS) to fluorescent product, 4-MU (Figure 2). The assay mixtures contained: For beta-G: For AS: 0.65 ml water 0.5 ml 0.1 M Tris buffer, pH 7 0.25 ml 0.01 mg/ml 4-MUG in water 0.1 ml 0.001 mg/ml beta-G in Tris (0 mg/ml beta-G/Tris as control) 1 ml water 1.7 ml 0.1 M Tris buffer, pH 7 0.2 ml 0.5 mg/ml 4-MUS in water 0.1 ml 0.01 mg/ml AS in Tris (0 mg/ml AS/Tris as control) For semiquantitative studies, fluorescence was detected by long-wave UV lamp (Black- Light Eastern Corporation, Model XX 15). The effects of inhibitors on the enzymatic reactions were assessed by substituting aqueous solutions of test inhibitor, at 0.1 mM to 100 mM, for the water. To rule out quenching of the fluorescence reaction by the inhibitors, separate tests were performed comparing the fluorescence from 4-MU at the maximum concentration expected in the enzyme reaction mixtures with either inhibitor solution or water. QUANTITATIVE ASSAYS OF BETA-G AND AS Several inhibitors of each, beta-G and AS, were assayed by quantitative fluorimetry. Studies were carried out with a Perkin-Elmer model MPF-3 with an Osram XBO high pressure Xenon lamp, using quartz curettes only. Prior to the performance of quantita- tive assays it was necessary to determine absorption and emission maxima of the fluores-
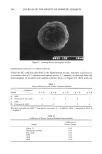
178 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS cent product 4-MU, as well as a concentration range for which the fluorescence inten- sity of this species varies linearly with concentration. A solution of 4-MU, 2 •g/ml, was prepared in 0.1 M Tris buffer, pH 7.0. The absorption spectrum was scanned, giving a maximum at 334 nm. The emission maximum, 444 nm, was obtained by scanning the emission spectrum while irradiating at 334 nm. All quantitative fluo- rescence studies employed these parameters. Linear calibration curves (Figure 4) were generated for the concentration ranges, (1) 0.2 •g/ml-2.0 •g/ml and (2) 0.02 •g/ml-0.2 •g/ml. The levels of 4-MU generated in beta-G and AS assays fall into range 1. The conversion factor was 1 fluorescence unit = 0.09 •M 4-MU. Samples were prepared as described above. All components (except the enzyme) were warmed to 37øC before addition of the enzyme. During the assays, reaction mixtures were maintained at 37øC. Fluorescence intensities were measured as a function of time. ODOR PRODUCTION IN APOCRINE SECRETION Sterile, odorless apocrine secretion was eluted from capillaries into 1.5 ml of 0.1 M Tris buffer, pH 7.0. In the first study, apocrine sweat was treated with beta-glucuronidase 12 10 o o.o ! 4-MU concentration, ug/ml Figure 4. Fluorescence as a function of 4-methylumbelliferol concentration. 0.2
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)