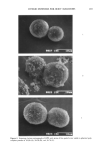
AXILLARY MALODOR 179 (E. coli) and aryl sulfatase (Aerobacter aerogenes) at 0.01 mg/ml in Tris buffer, 0.1 M, pH 7.0. The apocrine secretion was also treated with the lipophilic diphtheroid and with a mixed culture of axillary bacteria, each in sterile saline suspension. In the second study, inhibitors of the two enzymes were included in the reaction mix- tures--Zn + + as ZnGlycinate, saccharic acid lactone, and hexametaphosphate. Samples were prepared in gas chromatography vials and sealed with crimp caps. Samples were incubated at 37øC. In the first study, odor evaluations were conducted at 16 hr and 40 hr of incubation. In the second study, odor evaluations were conducted at 24 hr and 48 hr. Odor evaluations were performed by a panel of five, including one professional perfumer. ENZYME ACTIVITY IN CELL-FREE MEDIA Lipophilic diphtheroid was grown for 18 hr in brain-heart infusion containing 0.1% Tween 80, centrifuged for 20 min, and the supernatant medium filtered through a 0.22-micron filter. The substrate solution (2 ml of 4-MUG or 4-MUS) was mixed with 1 ml of the filtered medium (or water, as control). RESULTS PILOT STUDY A double-blind screening study was designed to detect the enzymes in axillary secre- tions. On plates designed to test for beta-G, ten sweat samples gave high fluorescence nine of these came from "high-odor formers." Only one came from a "low-odor former." On plates designed to test for AS, six of eight "high-odor formers" were found to have swabbings containing AS (two samples were lost), and only two of the ten "low-odor formers" had the enzyme. This study shows that at least some axillary bac- teria generate steroid esterases and that the presence of these enzymes may be correlated with axillary odor. SEMIQUANTITATIVE ASSAYS OF ENZYMES: EFFECTS OF INHIBITORS Various inhibitors of mammalian beta-Gs and ASs have been reported in the literature (18-32). We tested several of these compounds as well as related materials in our assay. The classic inhibitor of beta-G is saccharic acid-A-lactone (glucarolactone) (24). We tested this material as well as the simple sugars, mannose, fucose, and galactosamine, and the polysaccharide pullulan. One of the reported inhibitors of beta-G, EDTA (21), is a strong chelating agent. Thus we also tested o-phenanthroline, citrate, gluconate, nitrilotriacetate, and hexametaphosphate against the enzyme. Table I summarizes the materials tested. Several materials were found to inhibit each enzyme, but three of the agents tested-- Zn + +, Cu + +, and hexametaphosphate--inhibited both beta-G and AS. QUANTITATIVE ASSAYS OF BETA-G AND AS The effects of five inhibitors of beta-G and three inhibitors of AS identified in semi-
180 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table I Compounds Tested for Inhibition of Aryl Sulfatase and beta-Glucuronidase Enzyme inhibition For beta G: Cu + + Zn + + SPORIX D-glucaro-A-lactone EDTA NTA O-phenanthroline Citrate Sodium sulfate Gluconate Mannose Fucose Galactosamine Pullulan For AS: Cu + + Zn + + SPORIX hexametaphosphate Orthophosphate EDTA NTA quantitative assays were studied quantitatively in a fluorescence assay. As in the semi- quantitative tests, the progress of the reactions was indicated by the development of fluorescent product 4-MU from the non-fluorescent substrates, 4-MUG and 4-MUS. The data was recorded as fluorescence emission intensity as a function of time. A repre- sentative example of the results of these assays is given in Figure 5, which shows the effect of glucarolactone concentration upon beta-G activity. Similar plots were con- structed for each inhibitor. At each concentration, the beginning slope indicated initial reaction rate. These rates were then expressed as a fraction of the uninhibited reaction rate and plotted against the log of the inhibitor concentration (Figures 6 and 7). For beta-G (Figure 6) the glucarolactone was the most effective inhibitor (1-10 range). The divalent cations, Zn + + and Cu ++, were effective at approximately 10-100 IxM. The sequestering agents EDTA and phenanthroline were significantly less effective, providing inhibition at millimolar levels. For AS, the Cu + + ion was most effective (0.1-10 IxM range). Zn + + and phosphate were effective at 10- 100 I. tM. Most microbial AS belongs to the Type I class, which is not inhibited significantly by sulfate or phosphate, but the AS of Aerobacter aerogenes (our enzyme) is somewhat more sensitive than that of other bacteria (30). This property is apparent in our study, where phosphate is found to be about as effective as zinc cation. These studies show that at least some bacterial beta-G and AS can be inhibited by Zn, Cu, chelating agents, or glucarolactone.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)