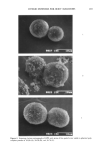
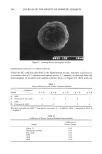
AXILLARY MALODOR 18 5 (14) (15) (16) (17) (18) (19) (20) (21) (22) (23) (24) (25) (26) (27) (28) (29) (30) (31) (32) R. Hobkirk, Steroid sulfotransferases and steroid sulfate sulfatases: Characteristics and biological roles, Can. J. Biochem., 63, 1127-1144 (1985). P. M. Sokolove, M. A. Wilcox, R. G. Thurman, and F. C. Kauffman, Stimulation of hepatic micro- somal beta-glucuronidase by calcium, Biochem. Biophys. Res. Commun., 121, 987-993 (1984). R. W. Trepeta and S.C. Edberg, Methylumbelliferyl-beta-D-glucuronide-based medium for rapid isolation and identification of Escherichia coli, J. Clinical Microbiol., 19, 172-174 (1984). J. V. Dubsky and A. Rabas, The formation of salts with glycine, Spisy Vydavane Prirodoveckedou Fakultou Masarykovy Univ. No. 115, 12-6 [Chem. Abs. 24, 4722 (1931)]. M. L. Doyle, P. A. Katzman, and E. A. Doisy, Production and properties of bacterial beta-glucuron- idase, J. Biol. Chem., 217, 921-930 (1955). V. Graef, E. Furuya, and O. Nishikaze, Hydrolysis of steroid glucuronides with beta-glucuronidase preparations from bovine liver, Helix pomatia, and E. coli, Clin. Chem., 23, 532-535 (1977). U. G. Ahlborg, E. Manzor, and T. Thunberg, Inhibitions of beta-glucuronidase by chlorinated hy- droquinones and benzoquinones, Arch. Toxicol., 37, 81-87 (1977). J. Kushari and M. Mukherjea, Studies on beta-glucuronidase of the developing human placenta, Gynecol. Obstet. Invest., 11, 119-127 (1980). J. E. Christner, S. Nand, and N. S. Mhatre, The reversible inhibition of beta-glucuronidase by organic peroxides, Biochem. Biophys. Res. Commun., 38, 1098-1104 (1970). T. Niwa, T. Tsuruoka, S. Inoue, Y. Naito, T. Koeda, and T. Niida, A potent new beta-glucuroni- dase inhibitor of D-glucaro-delta-lactam derived from nojirimycin,J. Blochem. (Tokyo), 72, 207-211 (1972). T. Okhubo and S. Sano, Mechanism of the action of beta-glucuronidase inhibitor upon apocrine sweat and sebacious glands and its dermatological application. Acta Dermatovener. (Stockholm), 53, 85-93 (1974). L. H. Chen and L. V. Packerr, Influence of alpha-tocopherol on the inhibition of beta-glucuronidase by peroxidized linoleic acid, Am. J. Clin. Nut., 24, 1232-1237 (1971). E. R. Schwartz and L. Adamy, Effect of ascorbic acid on aryl sulfatase activities and sulfated proteo- glycan metabolism in chondrocyte cultures, J. Clin. Invest., 60, 96-106 (1977). E. R. Schwartz, Effects of vitamins C and E on sulfated proteoglycan metabolism and sulfatase and phosphatase activities in organ cultures of human cartilage, Calcified Tissue International, 28, 201-208 (1979). A. L. Fluharry, R. L. Stevens, R. T. Miller, S. S. Shapiro, and H. Kihara, Ascorbic acid-2-sulfate sulfohydrolase activity of human arylsulfatase A, Biochim. Biophys. Acta, 429, 508-516 (1976). L. R. Fowler and D. H. Rammler, Sulfur metabolism of Aerobacter aerogenes. II. The purification and some properties of a sulfatase, Biochemistry, 3, 230-237 (1964). K. S. Dodgson, B. Spencer, and K. Williams, Examples of anti-competitive inhibition, Nature, 177, 432-433 (1956). D. Robinson, J. N. Smith, B. Spencer, and R. T. Williams, Studies in detoxification. 43. Aryl sulfatase activity of takadiastase towards some phenolic ethereal sulfates, Blochem. J•, 51, 202-208 (1952). A. B. Roy, Sulfatase of ox liver. XIII. Action of carbonyl reagents on sulfatase A, Biochim. Biophys. Acta, 198, 76-81 (1970).
j. Soc. Cosmet. Chem., 41, 187-195 (May/June 1990) Chronic ultraviolet radiation-induced skin tumors and associated changes in basal epidermal ornithine decarboxylase activity GREGG. HILLEBRAND, MARCIA S. WINSLOW, DAVE A. HEITMEYER, and DONALD L. BISSETT, The Procter & Gamble Company, Miami Valley Laboratories, Cincinnati, OH 45239-8707. Received April 13, 1990. Synopsis The association between ultraviolet radiation (UVR)-induced skin neoplasia and the basal levels of the epidermal enzyme ornithine decarboxylase (ODC) was studied in Skh:HR-1 hairless mice. Groups of mice were chronically exposed to UVR (15 weeks), then treated with photoprotective agents (12-20 weeks) with or without continued UVR exposure. After treatment, total skin papilloma/carcinoma (tumor) area per mouse and epidermal ODC activity (in the treated and UVR-exposed but non-tumor-involved dorsal skin) were measured in each group. Mice with the largest average tumor area had substantially elevated basal ODC activities (•350-fold) relative to non-irradiated control mice, which lacked tumors. Groups of mice treated with photoprotective agents showed intermediate levels of skin tumors and correspondingly inter- mediate epidermal ODC activities. Basal epidermal ODC activity was also measured in lifetime sun-exposed and unexposed skin of four healthy human volunteers with no history of skin cancer. No significant relationship was observed between human epidermal ODC activity and prior history of solar exposure in these individuals. These human data are consistent with recent hairless mouse studies [Hillebrand, G. G., Winslow, M. S., Benzinger, M. A., Heirmeyer, D. A., and Bissett, D. L., Cancer Res., 50, 1580-1584 (1990)] showing that chronically irradiated mice lacking visible tumors had normal levels of epidermal ODC activity. The results support the idea that elevated levels of epidermal ODC activity may be specifically indicative of chronic UVR-in- duced neoplastic growth. INTRODUCTION The visible appearance of skin, especially facial skin, can be very dependent on an individual's past history of solar radiation exposure. For example, chronic solar radia- tion exposure can lead to damage of the underlying dermal connective tissue (1-4), manifested as accelerated visible skin aging in the form of wrinkles (5-8). In more severe cases, chronic photodamage can progress to the most common forms of skin cancer, i.e., basal and squamous cell carcinoma (9). The diagnosis and development of therapy for photodamaged skin is facilitated by the employment of biochemical markers 187
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)