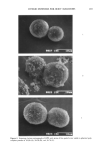
AXILLARY MALODOR 183 Neither apocrine sweat nor either of the pure enzymes exhibited odor. Cultures of lipophilic diphtheroid, and a mixed axillary population, had the faint-to-moderate malodor characteristic of any bacterial culture, but no particular "sweaty" odor. How- ever, when apocrine sweat was mixed with either enzyme, or with either bacterial culture, a strong, sweaty odor was produced. To confirm and characterize the implication of lipophilic diphtheroids, the enzyme substrates were exposed to cell-free culture medium. The medium exhibited both aryl sulfatase and beta-glucuronidase activity. The fact that the odor can be produced by the enzymes alone indicates that it is their activity that is the essential cause of steroidal axillary malodor, confirming the hy- pothesis from the preliminary tests above. The fact that the odor is produced by lipo- philic diphtheroids alone suggests that this strain may be one member of the population that produces the enzymes, and accounts for its implication in odor production by Leyden et al. (6) and Jackman et al. (5). The presence of enzyme activity in cell-free diphtheroid medium shows that the enzymes are extracellular. Any subsequent intra- cellular enzymatic conversions of the steroids that may occur seem not to be essential to odor production. Because the sterile secretion alone generated a faint musky odor after incubation at 37 ø C, we tested it for indigenous beta-G and AS activity: Addition of 4-MUG, the beta-G substrate, produced the characteristic 4-MU fluorescence, but addition of 4-MUS, the AS substrate, had no effect. The presence of endogenous beta-G in the apocrine gland has been noted separately by Ohkubo and Sano (24). INHIBITION OF ODOR FROM APOCRINE SECRETION Results of the second study are presented in Table III. Again, odor was generated only in those samples containing either apocrine secretion with enzymes or apocrine secretion with bacteria. Zinc glycinate was highly effective in preventing odor the odor-inhib- iting effect of the lactone, although significant, was weaker than that of ZnGLY in both systems the polyphosphate (SPORIX) had no effect. (It is known that polyphosphates are hydrolyzed in aqueous solution. Possibly SPORIX was degraded to inactive frag- ments during incubation it is one of the weakest beta-G inhibitors in Table I.) Table III In Vitro Deodorant Action of Enzyme Inhibitors Enzymes Apocrine sweat Enzymes + apocrine sweat Enzymes + sweat + ZnGLY Enzymes q- sweat q- SPORIX Enzymes + sweat + lactone Lipophilic diphrheroids LDs q- apocrine sweat LDs + sweat + ZnGLY LDs + sweat + lacrone No odor No odor to faint off-odor Faint/moderate musky-sweaty No odor Faint/moderate musky-sweaty No odor to faint musky-sweaty Faint off-odor Strong, sweaty Faint stale musky Moderate sweaty
184 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS DISCUSSION It has long been recognized that axillary odor arises from the interaction of bacteria with apocrine sweat. A class of volatile free steroids has been associated with axillary odor and with a particular bacterial population--the lipophilic diphtheroids. We suspected that the enzymes responsible for this process would be those capable of releasing free volatile steroid from odorless steroid conjugates present in sterile apocrine secretion. We showed that a mixed culture of axillary bacteria produces a beta-glucuronidase capable of cleaving asteroid glucuronide. From our preliminary screening tests, we showed that another class of hydrolytic enzyme, aryl sulfatase, was also present in axil- lary strains. We have shown that a beta-glucuronidase and an aryl sulfatase, both of bacterial origin, will cleave odorless compounds in sterile secretion to produce distinct axillary odor. Thus, hydrolytic enzymes are implicated in the release of odor. We have also shown that a lipophilic diphtheroid secretes both of the enzymes necessary to produce steroid malodor from sterile axillary secretion. We have shown that the generation of odor, from the addition of beta-glucuronidase or aryl sulfatase or lipophilic diphtheroid to apocrine secretion, may be prevented by the inclusion of the enzyme inhibitor Zn + + and somewhat reduced by glucarolactone (which inhibits only the beta-glucuronidase but not aryl sulfatase). This further sub- stantiates the role of these bacterial enzymes in axillary odor. REFERENCES (1) (2) (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) (13) E. Eigen, A new mechanism of axillary malodor [Letter], J. Soc. Cosmet. Chem., 41, 147 (1990). J. S. Strauss and A. M. Kligman, The bacteria responsible for axillary odor. I., J. Invest. Dermatol., 27, 67-71 (1956). N. Shehadeh and A.M. Kligman, The bacteria responsible for axillary odor. II., J. Invest. Dermatol., 41, 3 (1963). J. N. Labows, K. J. McGinley, and A.M. Kligman, Perspectives on axillary odor, J. $oc. Cosmet. Chem., 34, 193-202 (1982). P. J. H. Jackman and W. C. Noble, Normal axillary skin microflora in various populations, Clin. Exp. Dermatol., 8, 259-268 (1983). J. J. Leyden, K. J. McGinley, E. Holzle, J. N. Labows, and A. M. Kligman, The microbiology of the human axilla and its relationship to axillary odor, J. Invest. Dermatol., 77, 413-416 (1981). R. R. Marples and A.M. Kligman, In-vivo methods for appraising anti-bacterial agents, TGA Cosmet. J., 1, 26-33 (1969). B. W. L. Brooksbank, R. Brown, and J.-A. Gustafsson, The detection of 5 alpha-androst-16-en-3 alpha ol in human male axillary sweat, Experientia, 30, 864-865 (1964). A. Nixon, A. I. Mallet, and D. B. Gower, Simultaneous quantification of five odorous steroids (16-androstenes) in the axillary hair of men, J. Steroid Blochem., 29, 505-510 (1988). S. Bird and D. B. Gower, Axillary 5-alpha-androst-16-en-3-one, cholesterol, and squalene in men: Preliminary evidence for 5-alpha-androst-16-en-3-one being a product of bacterial action, J. Steroid Blochem., 17, 517-522 (1982). J. N. Labows, G. Preti, E. Holzle, J. J. Leyden, and A. M. Kligman, Steroid analysis of human apocrine secretion, Steroids, 34, 249-258 (1979). W. C. Noble, Microbiology of Human Skin. (Lloyd-Duke Medical Books, London, 1981) pp. 92-106. A. Nixon, A. I. Mallet, P. J. H. Jackman, and D. B. Gower, Testosterone metabolism by isolated human axillary corynebacterium ssp.: A gas-chromatographic mass-spectrometric study, J. Steroid Blochem., 27, 1-6 (1986).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)