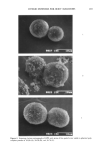
HYBRID POWDERS FOR BODY MALODORS 207 CONCLUSIONS Hybridization of spherical polyethylene powder and fine-particle zinc oxide or fine-par- ticle aluminum chlorohydrate resulted in hybrid powders that possessed the advantages of both the core and the outer layer powders. The texture of fine-particle zinc oxide and fine-particle aluminum chlorohydrate im- proved drastically when hybridized with spherical polyethylene powder. The results obtained from the direct evaluation method and instrumental evaluation method were in agreement. The quenching power of zinc oxide was completely retained and, in the case of alu- minum chlorohydrate, was even enhanced when hybridized. In this study we have emphasized the effectiveness of hybrid powders as quenching actives to eliminate body malodors. Numerous variations of hybrid powders can be achieved by changing the core powder and the outer layer powder. This hybridization technology we believe will be applied widely in the field of cosmetics and pharmaceu- ticals in the future. REFERENCES (1) T. Nakane, Y. Yahata, K. Yoshida, and T. Nanba, Hybrid fine powder, Boundary, 1, 28-30 (1985). (2) T. Nakane, T. Nanba, and K. Tomira, Development and functions of hybrid powders by mechano- chemical methods, 2 6th S C CJ C onj•rence Preprints, 17-18 (1989). (3) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, O. Nakata, and Y. Fujiyama, Elucidating body malodour to develop a novel body odour quencher, 15th 1FSCC International Congress Preprints, 3, 529-562 (1988). (4) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, O. Nakata, and Y. Fujiyama, Elucidating body malodour to develop a novel body odour quencher, J. Soc. Cosmet. Chem. Japan, 23, 217-224 (1989). (5) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, and O. Nakata, Elucidation of chemical compounds responsible for foot malodour, Brit. J. Dermatol., 122, 771-776 (1990). (6) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, and O. Nakata, Development of a novel hybrid powder formulated to quench body odor, J. Soc. Cosmet. Chem., 40, 335-346 (1989). (7) Y. Fujiyama and F. Suzuki, Cosmetics and powder technology, Funtai Ko-gaku-kaishi, 9, 565-573 (1984).
J. Soc. Cosmet. Chem., 41, 209-212 (May/June 1990) A note on the permanent setting of human hair MAX FEUGHELMAN, School of Fibre Science and Technology, The University of New South Wales, Kensington, N.S.W. 2033, Australia. Received July 30, 1990. Synopsis In the standard permanent setting procedure for human hair, ammonium thioglycollate is applied to the curled hair. Disulfide bonds are converted to sulfhydril groups to enable the protein structure of the hair fibers to relax mechanically by the mechanism of sulfhydril disulfide interchange. After relaxation, the sulfhydril groups are reoxidized (neutralized) to reform the disulfide bonds, thus stabilizing the curled conformation. If the mechanical relaxation is carried out at an elevated temperature, the relaxation can occur with a much lower coversion of disulfide to sulfhydril groups. If at these elevated temperatures, the density of sulfhydril groups formed is low, returning the hair fibers to room temperature is sufficient to stabilize the curl. This eliminates the need for oxidation of the sulfhydril groups back to disulfides and results in major benefits of time saved and fiber degradation as observed by appearance and feel. Other benefits are also noted for the application of this technique to the permanent waving procedure. In the permanent waving of human hair the overall procedural steps normally applied are as follows: 1. The hair fibers are shaped into the proposed, i.e., wrapped on a normal perm-roller. A reducing agent that penetrates into the hair is applied. 2. The reducing agent results in the cleavage of the covalently bonded disulfides of the cystine bonds, which cross link the protein chains forming the hair fibers. The cleavage results in the formation of two sulfhydril groups from every disulfide group reduced. 3. With the cleavage of the cystine bonds, the protein chains of the keratin structure of the hair fibers are able to rearrange to reduce or remove the forces present in the structure (which would tend to return each fiber to its original configuration on being released from the perm-roller). 4. The arrangement of the protein chains, which has occured in the curled fibers being set, is stabilized by reforming the cystine bonds through the use of a suitable oxi- dizing agent (the neutralizer). It is well recognized that the time of application of the waving solution consisting of a reducing agent such as ammonium thioglycollate, depends on the pH and temperature. What is not emphasized, is that the cleavage of the disulfide bonds is not the prime factor in the rate of rearrangement of the protein chains. The rate is primarily depen- dent on the proportion of ionized sulfhydril groups present, which enables the protein 209
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)